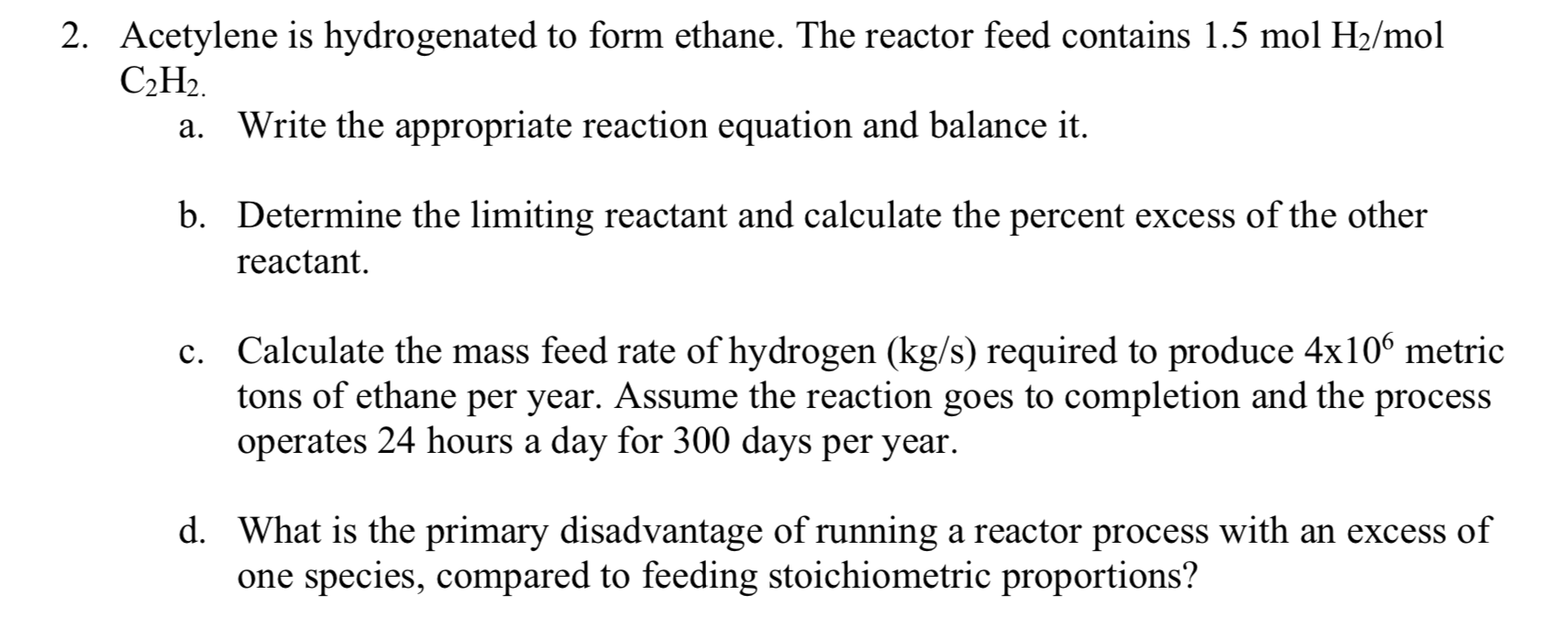
Acetylene Is Hydrogenated To Form Ethane - Acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h2/mol ch2. (a) the stoichiometric reaction of hydrogenation of acetylene to ethane is represented by the equation c2h2 + 2h2 →. Draw and label the flowchart. The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: The balanced equation for the hydrogenation of acetylene to form ethane is: The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The feed to the reactor contains 1.50 mol h2/mol c2h2. Acetylene (c2h2) is hydrogenated to form ethane (c2h).
The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The balanced equation for the hydrogenation of acetylene to form ethane is: Draw and label the flowchart. Acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h2/mol ch2. The feed to the reactor contains 1.50 mol h2/mol c2h2. (a) the stoichiometric reaction of hydrogenation of acetylene to ethane is represented by the equation c2h2 + 2h2 →. Acetylene (c2h2) is hydrogenated to form ethane (c2h). The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as:
The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The feed to the reactor contains 1.50 mol h2/mol ch2. (a) the stoichiometric reaction of hydrogenation of acetylene to ethane is represented by the equation c2h2 + 2h2 →. Draw and label the flowchart. The balanced equation for the hydrogenation of acetylene to form ethane is: Acetylene (c2h2) is hydrogenated to form ethane (c2h). Acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h2/mol c2h2. The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as:
Acetylene is hydrogenated to form ethane. The feed to the reactor
The feed to the reactor contains 1.50 mol h2/mol ch2. Draw and label the flowchart. (a) the stoichiometric reaction of hydrogenation of acetylene to ethane is represented by the equation c2h2 + 2h2 →. Acetylene is hydrogenated to form ethane. Acetylene (c2h2) is hydrogenated to form ethane (c2h).
Solved Acetylene is hydrogenated to form ethane. The feed to
The balanced equation for the hydrogenation of acetylene to form ethane is: Acetylene (c2h2) is hydrogenated to form ethane (c2h). The feed to the reactor contains 1.50 mol h2/mol c2h2. The feed to the reactor contains 1.50 mol h2/mol ch2. (a) the stoichiometric reaction of hydrogenation of acetylene to ethane is represented by the equation c2h2 + 2h2 →.
Solved Acetylene (C2H2(g)) is hydrogenated to form ethane
Draw and label the flowchart. The feed to the reactor contains 1.50 mol h2/mol ch2. Acetylene is hydrogenated to form ethane. Acetylene (c2h2) is hydrogenated to form ethane (c2h). The balanced equation for the hydrogenation of acetylene to form ethane is:
Solved 2. Acetylene is hydrogenated to form ethane. The
Acetylene (c2h2) is hydrogenated to form ethane (c2h). The balanced equation for the hydrogenation of acetylene to form ethane is: The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: The principle involved in the conversion of acetylene into ethane is known as hydrogenation. Acetylene is hydrogenated to form ethane.
Acetylene is hydrogenated to form ethane. The feed to the reactor
Acetylene is hydrogenated to form ethane. Draw and label the flowchart. The feed to the reactor contains 1.50 mol h2/mol c2h2. (a) the stoichiometric reaction of hydrogenation of acetylene to ethane is represented by the equation c2h2 + 2h2 →. The feed to the reactor contains 1.50 mol h2/mol ch2.
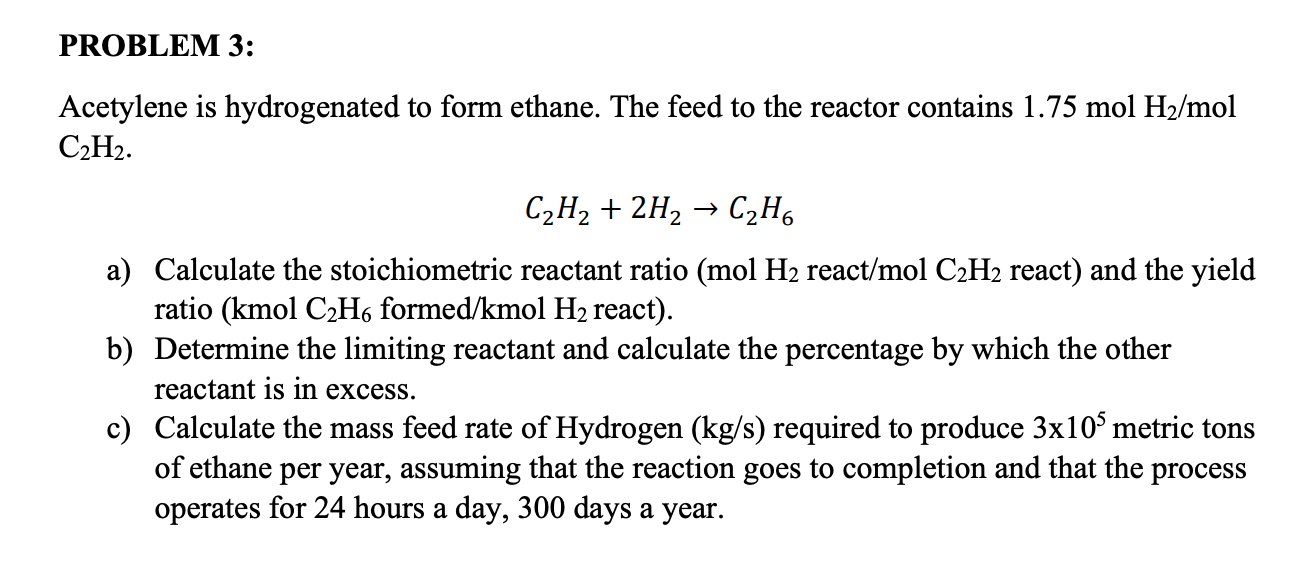
Solved PROBLEM 3 Acetylene is hydrogenated to form ethane.
The feed to the reactor contains 1.50 mol h2/mol ch2. The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The balanced equation for the hydrogenation of acetylene to form ethane is: The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: Acetylene is hydrogenated to form ethane.
Acetylene is hydrogenated to form ethane. The feed to the reactor
Acetylene is hydrogenated to form ethane. The balanced equation for the hydrogenation of acetylene to form ethane is: The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The feed to the reactor contains 1.50 mol h2/mol ch2.
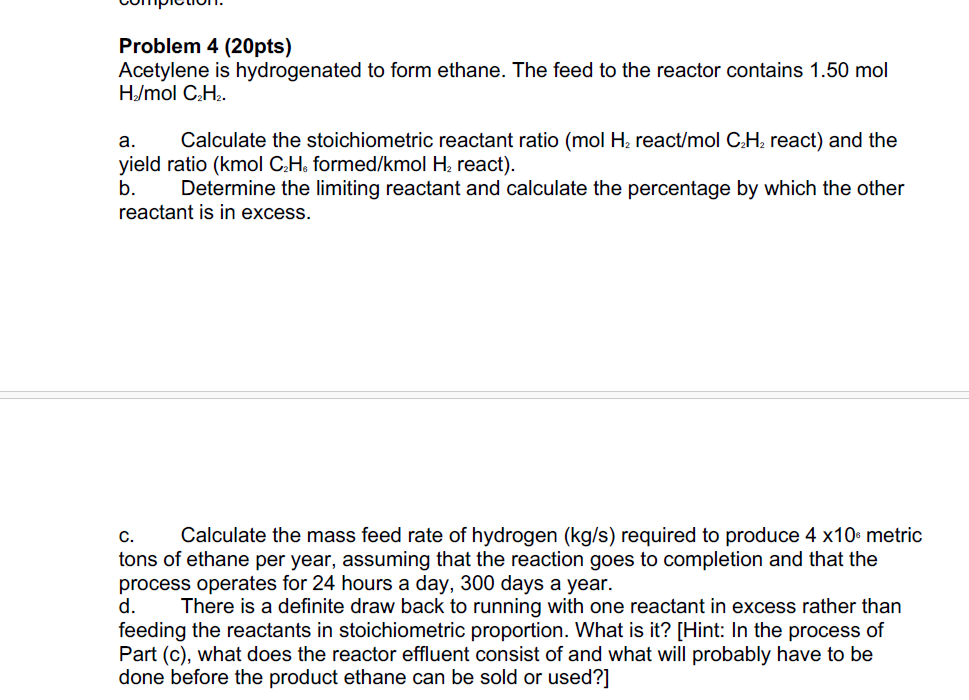
Solved Problem 4 (20pts)Acetylene is hydrogenated to form
The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The feed to the reactor contains 1.50 mol h2/mol c2h2. The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: Draw and label the flowchart. The feed to the reactor contains 1.50 mol h2/mol ch2.
Solved 4.52. Acetylene is hydrogenated to form ethane. The
The principle involved in the conversion of acetylene into ethane is known as hydrogenation. Draw and label the flowchart. The feed to the reactor contains 1.50 mol h2/mol c2h2. The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: Acetylene (c2h2) is hydrogenated to form ethane (c2h).
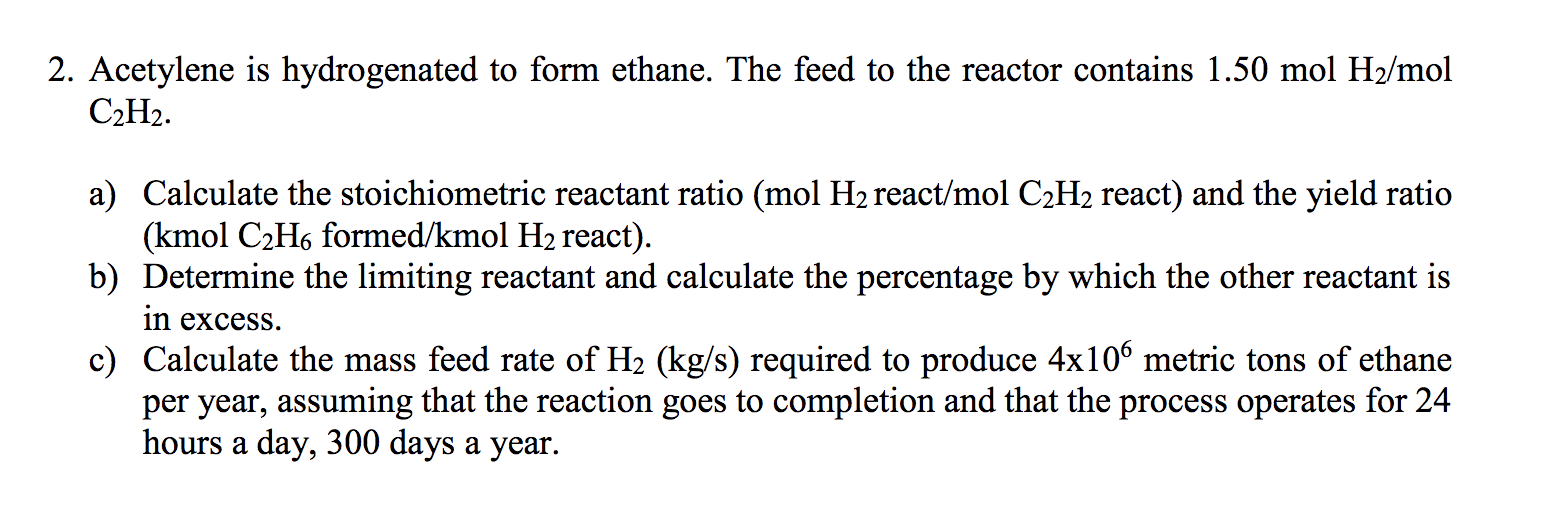
Solved 2. Acetylene is hydrogenated to form ethane. The feed
The feed to the reactor contains 1.50 mol h2/mol c2h2. The balanced equation for the hydrogenation of acetylene to form ethane is: The principle involved in the conversion of acetylene into ethane is known as hydrogenation. The feed to the reactor contains 1.50 mol h2/mol ch2. The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be.
The Reaction Between Acetylene (C2H2) And Hydrogen (H2) To Form Ethane (C2H6) Can Be Represented As:
Acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h2/mol ch2. The principle involved in the conversion of acetylene into ethane is known as hydrogenation. Draw and label the flowchart.
(A) The Stoichiometric Reaction Of Hydrogenation Of Acetylene To Ethane Is Represented By The Equation C2H2 + 2H2 →.
The balanced equation for the hydrogenation of acetylene to form ethane is: Acetylene (c2h2) is hydrogenated to form ethane (c2h). The feed to the reactor contains 1.50 mol h2/mol c2h2.