Atoms From Which Two Elements Would Form Ionic Bonds - Atoms from elements with significantly different electronegativities will. No, ionic bonds are formed between atoms of different elements that have. An ionic compound forms when elements form ionic bonds. Elements from group 1 or 2 will form ionic compounds with elements from. Group 16 elements (also known as the oxygen family) tend to gain two valence.
Atoms from elements with significantly different electronegativities will. No, ionic bonds are formed between atoms of different elements that have. Elements from group 1 or 2 will form ionic compounds with elements from. An ionic compound forms when elements form ionic bonds. Group 16 elements (also known as the oxygen family) tend to gain two valence.
Group 16 elements (also known as the oxygen family) tend to gain two valence. An ionic compound forms when elements form ionic bonds. Elements from group 1 or 2 will form ionic compounds with elements from. Atoms from elements with significantly different electronegativities will. No, ionic bonds are formed between atoms of different elements that have.
Atom Electrons, Nucleus, Bonds Britannica
Elements from group 1 or 2 will form ionic compounds with elements from. An ionic compound forms when elements form ionic bonds. Atoms from elements with significantly different electronegativities will. No, ionic bonds are formed between atoms of different elements that have. Group 16 elements (also known as the oxygen family) tend to gain two valence.
Examples of Ionic Bonds and Compounds
Group 16 elements (also known as the oxygen family) tend to gain two valence. An ionic compound forms when elements form ionic bonds. Elements from group 1 or 2 will form ionic compounds with elements from. No, ionic bonds are formed between atoms of different elements that have. Atoms from elements with significantly different electronegativities will.
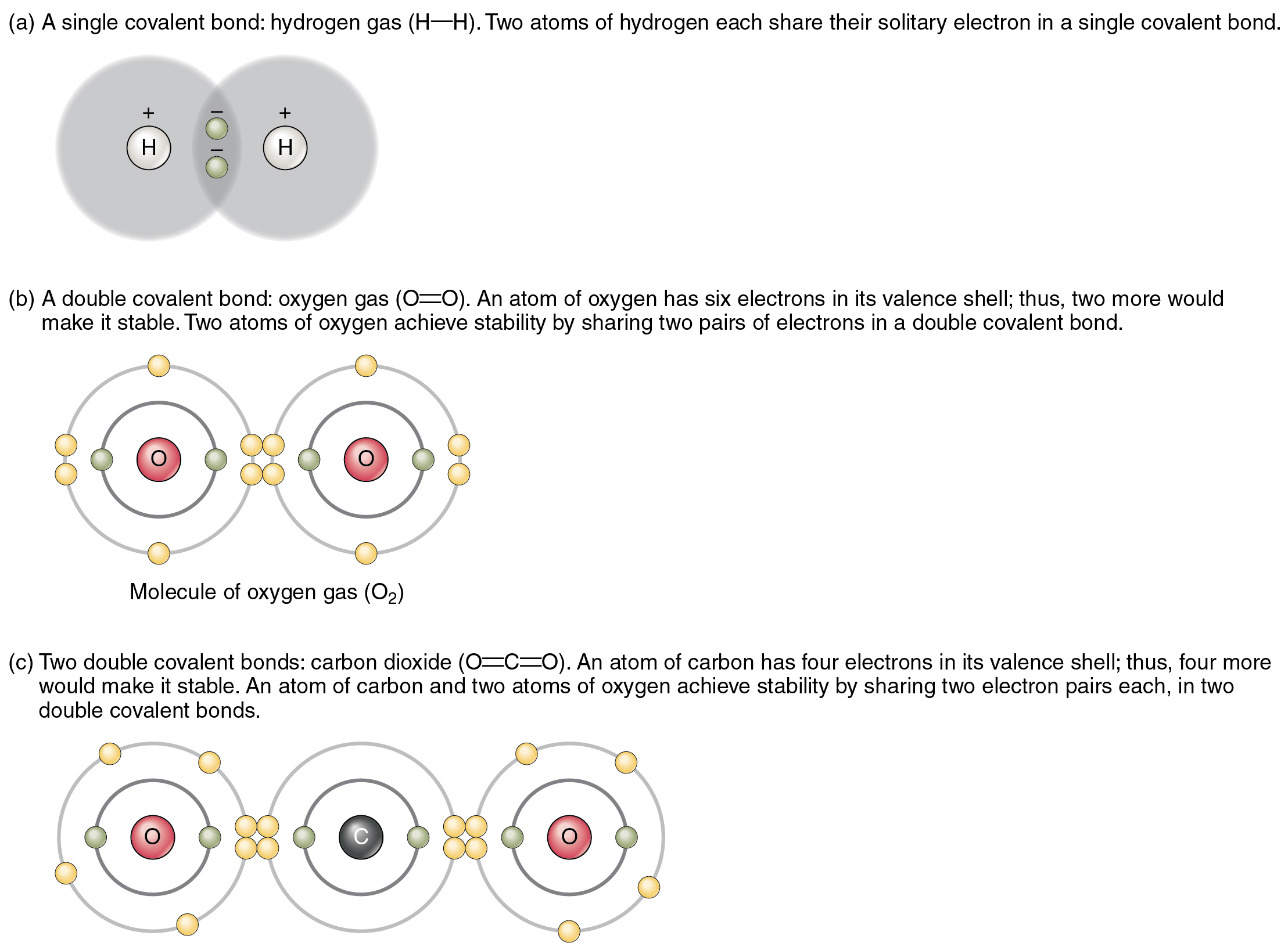
Chemical Bonds Form When Atoms
Group 16 elements (also known as the oxygen family) tend to gain two valence. Atoms from elements with significantly different electronegativities will. Elements from group 1 or 2 will form ionic compounds with elements from. No, ionic bonds are formed between atoms of different elements that have. An ionic compound forms when elements form ionic bonds.
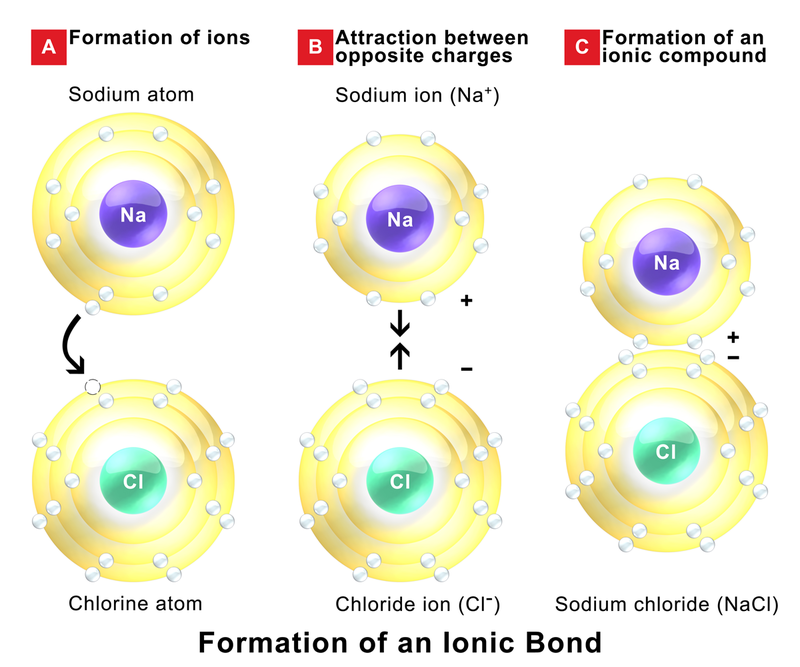
Ionic Bond — Formation & Compounds Expii
Elements from group 1 or 2 will form ionic compounds with elements from. Group 16 elements (also known as the oxygen family) tend to gain two valence. No, ionic bonds are formed between atoms of different elements that have. An ionic compound forms when elements form ionic bonds. Atoms from elements with significantly different electronegativities will.
Ionic Bonding (Biology) — Definition & Role Expii
An ionic compound forms when elements form ionic bonds. No, ionic bonds are formed between atoms of different elements that have. Elements from group 1 or 2 will form ionic compounds with elements from. Group 16 elements (also known as the oxygen family) tend to gain two valence. Atoms from elements with significantly different electronegativities will.
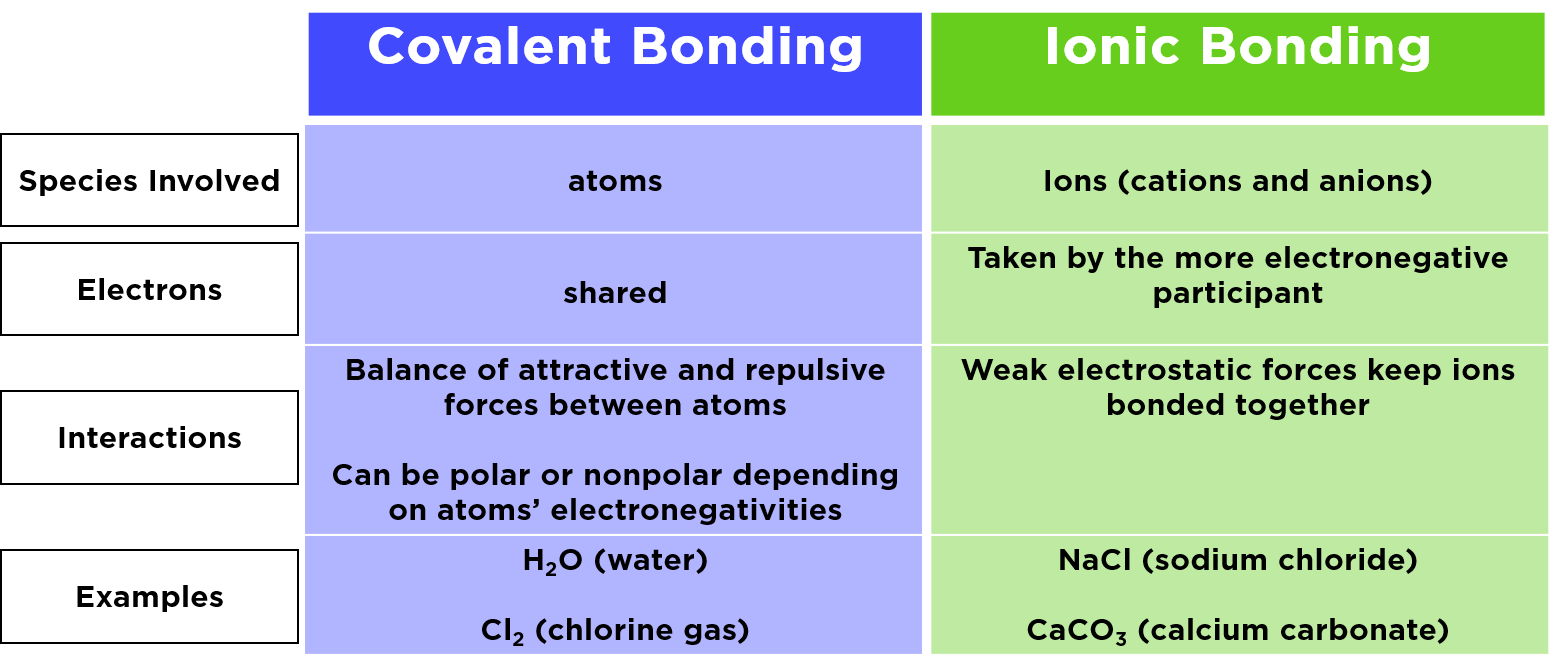
Difference Between Ionic Covalent and Metallic Bonds Definition
No, ionic bonds are formed between atoms of different elements that have. Elements from group 1 or 2 will form ionic compounds with elements from. Atoms from elements with significantly different electronegativities will. An ionic compound forms when elements form ionic bonds. Group 16 elements (also known as the oxygen family) tend to gain two valence.
Ionic bond Definition, Properties, Examples, & Facts Britannica
No, ionic bonds are formed between atoms of different elements that have. Atoms from elements with significantly different electronegativities will. An ionic compound forms when elements form ionic bonds. Elements from group 1 or 2 will form ionic compounds with elements from. Group 16 elements (also known as the oxygen family) tend to gain two valence.
Ionic Bond — Formation & Compounds Expii
Elements from group 1 or 2 will form ionic compounds with elements from. Atoms from elements with significantly different electronegativities will. No, ionic bonds are formed between atoms of different elements that have. Group 16 elements (also known as the oxygen family) tend to gain two valence. An ionic compound forms when elements form ionic bonds.
How To Form Ionic Bonds
Elements from group 1 or 2 will form ionic compounds with elements from. Group 16 elements (also known as the oxygen family) tend to gain two valence. No, ionic bonds are formed between atoms of different elements that have. Atoms from elements with significantly different electronegativities will. An ionic compound forms when elements form ionic bonds.
What Is An Ionic Bond Sciencing Ionic Bonding Ionic Chemical Bond
Elements from group 1 or 2 will form ionic compounds with elements from. An ionic compound forms when elements form ionic bonds. Group 16 elements (also known as the oxygen family) tend to gain two valence. No, ionic bonds are formed between atoms of different elements that have. Atoms from elements with significantly different electronegativities will.
Group 16 Elements (Also Known As The Oxygen Family) Tend To Gain Two Valence.
An ionic compound forms when elements form ionic bonds. Atoms from elements with significantly different electronegativities will. No, ionic bonds are formed between atoms of different elements that have. Elements from group 1 or 2 will form ionic compounds with elements from.





.PNG)