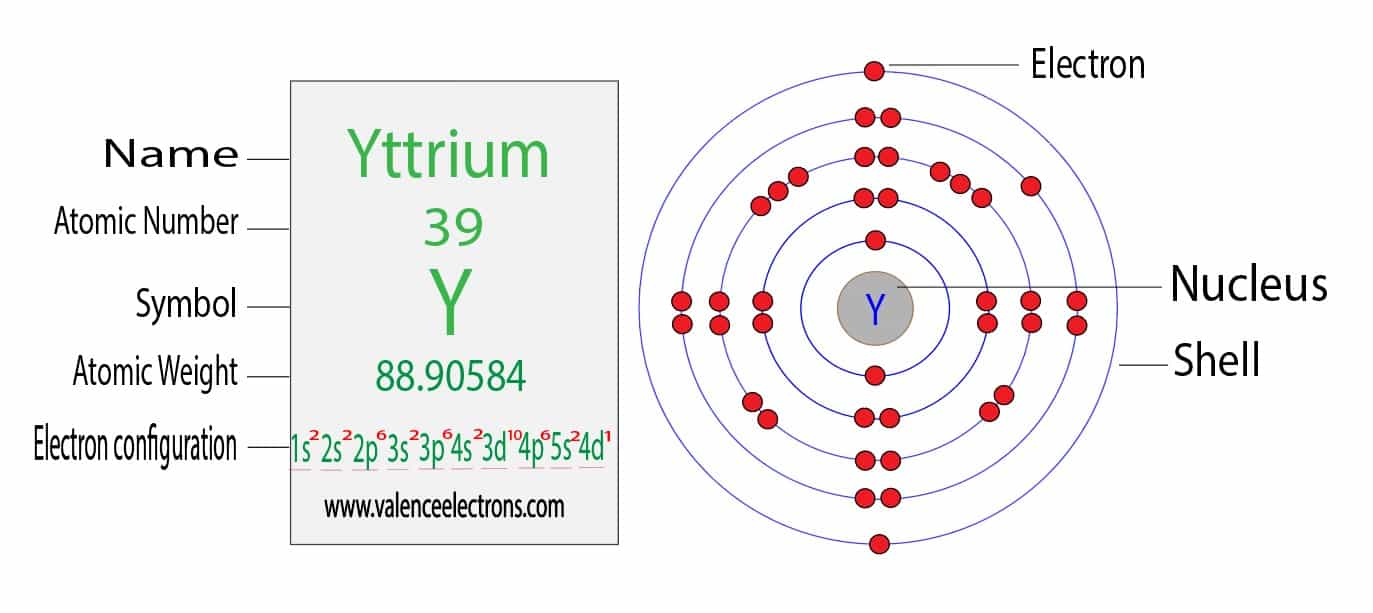
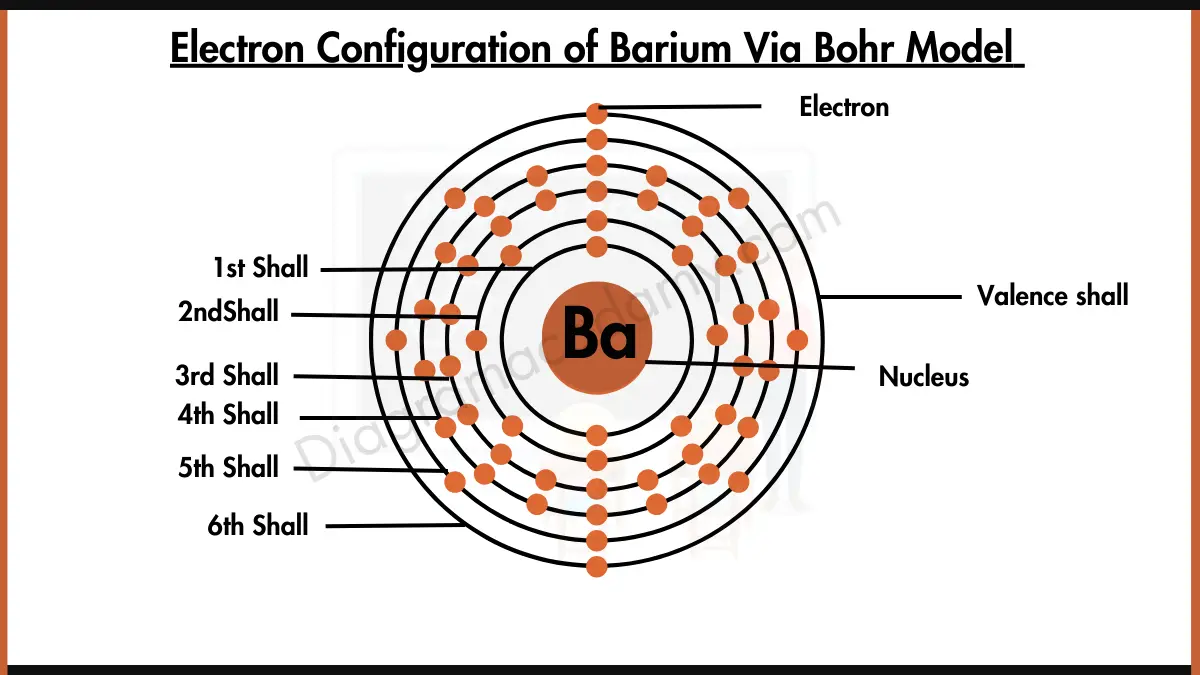
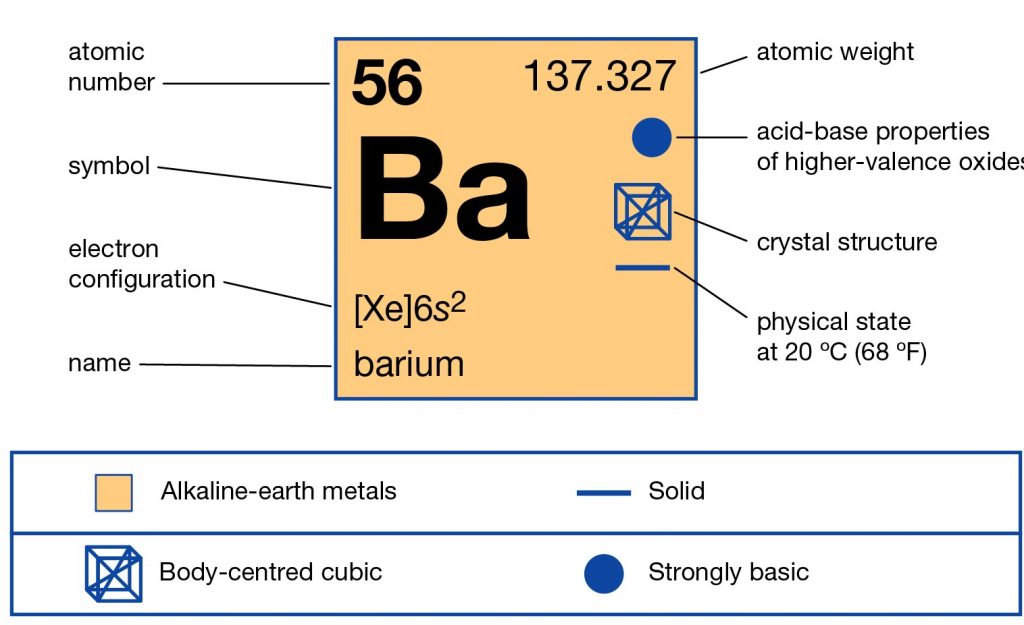
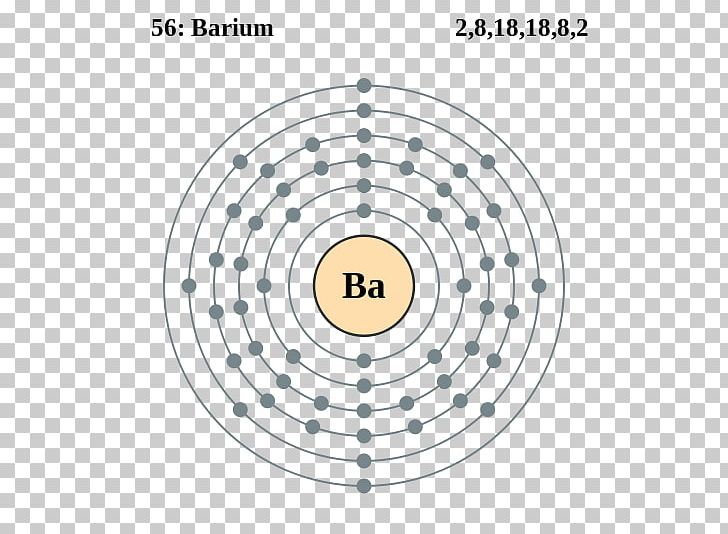
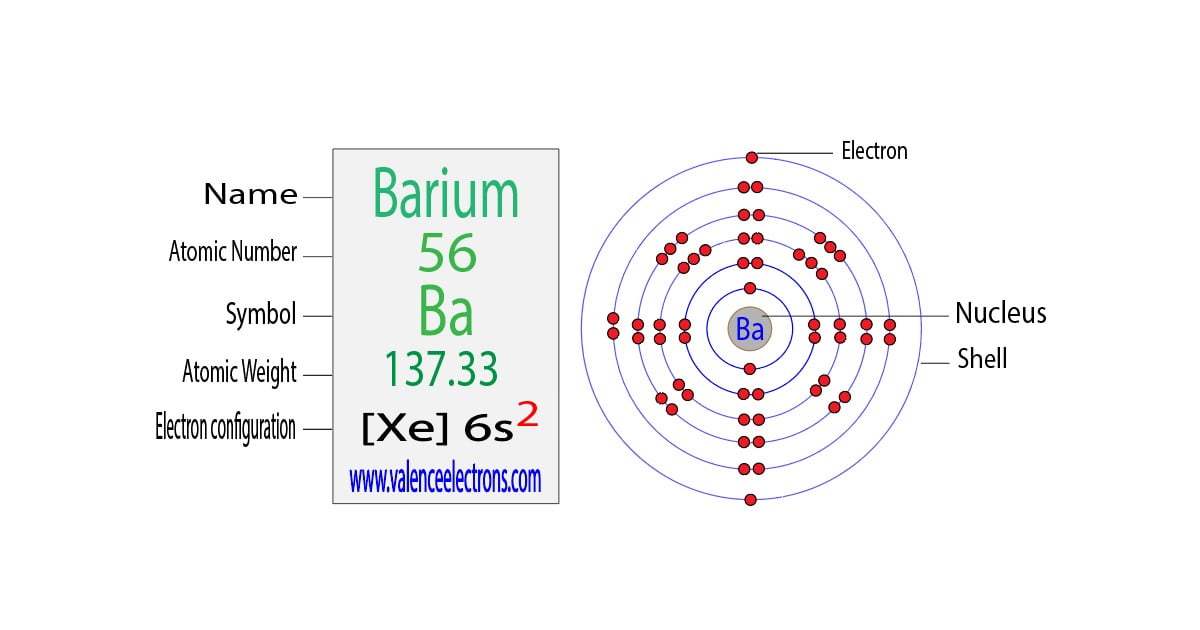
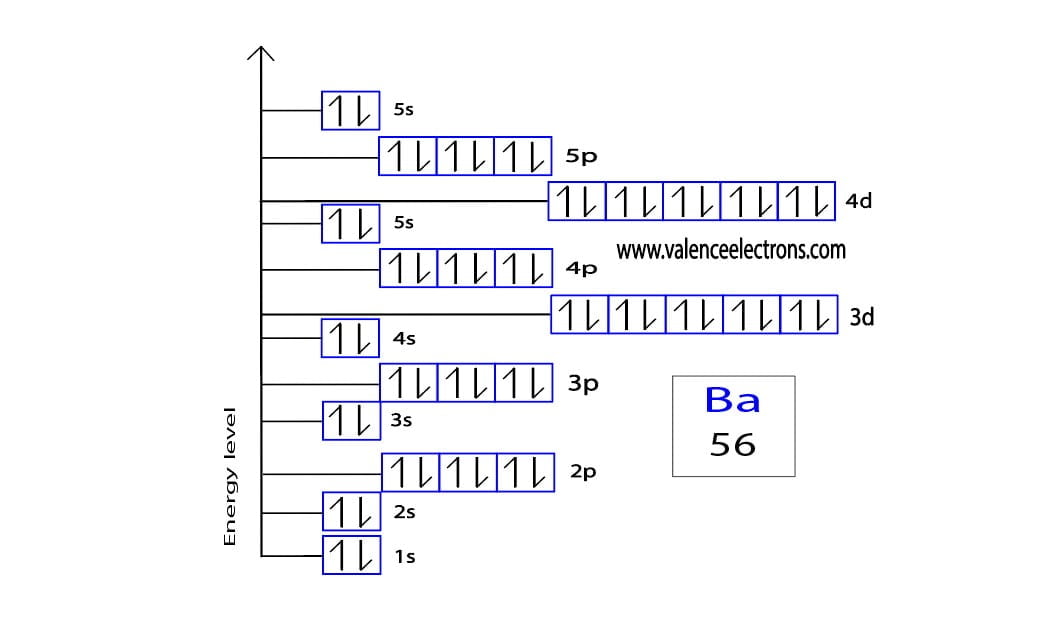
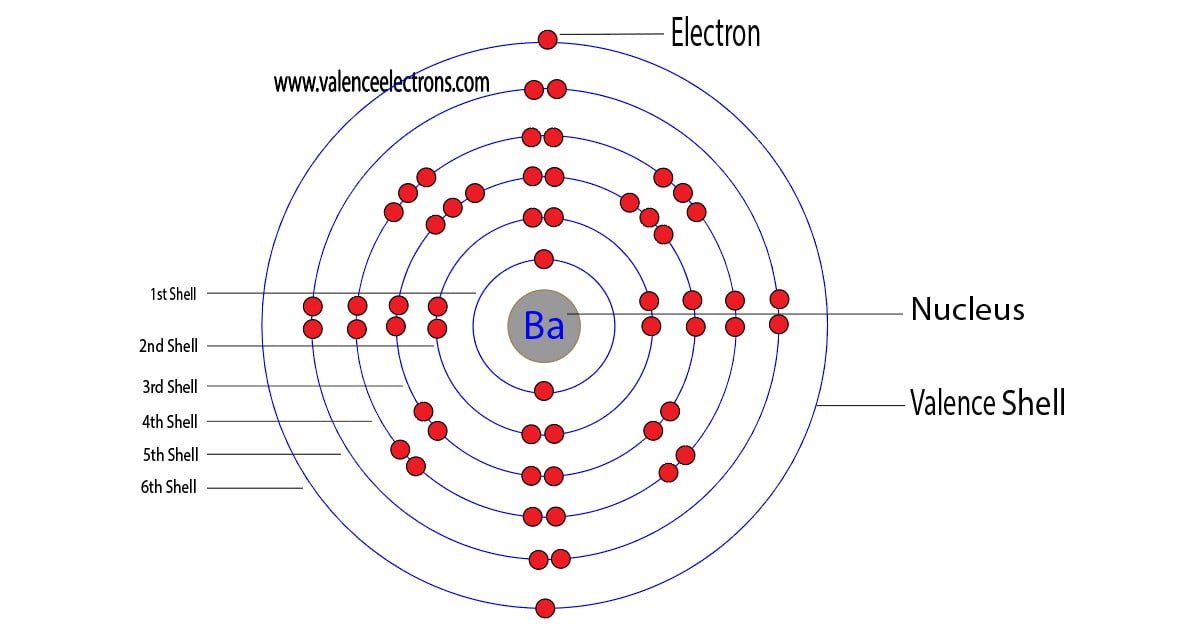
Barium Electron Configuration Long Form - Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Enter the atomic number or symbol of any element, including. Calculate the full and condensed electron configuration of barium (ba). Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2.
Enter the atomic number or symbol of any element, including. Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Calculate the full and condensed electron configuration of barium (ba). Full electron configuration of barium:
Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Full electron configuration of barium: Calculate the full and condensed electron configuration of barium (ba). 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Enter the atomic number or symbol of any element, including.
Electron Configuration Of Barium
Full electron configuration of barium: Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Calculate the full and condensed electron configuration of barium (ba). Enter the atomic number or symbol of any element, including. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10.
Barium Electron Configuratio(Explained for Beginners)
Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Calculate the full and condensed electron configuration of barium (ba). Enter the atomic number or symbol of any element, including. Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328.
Barium(Ba) Electron Configuration and Orbital Diagram
Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Calculate the full and condensed electron configuration of barium (ba). Enter the atomic number or symbol of any element, including. Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10.
Electron Configuration of Barium Diagram
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Calculate the full and condensed electron configuration of barium (ba). Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Enter the atomic number or symbol of any element, including. Full electron.
barium.. Dynamic Periodic Table of Elements and Chemistry
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Enter the atomic number or symbol of any element, including. Full electron configuration of barium: Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Calculate the full and condensed electron configuration.
Barium Electron Configuratio(Explained for Beginners)
Enter the atomic number or symbol of any element, including. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Calculate the full and condensed electron configuration of barium (ba). Full electron configuration of barium: Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328.
Electron Shell Barium Electron Configuration Atom PNG, Clipart
Enter the atomic number or symbol of any element, including. Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Calculate the full and condensed electron configuration of barium (ba). 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Full electron.
How to Write the Electron Configuration for Barium (Ba)
Calculate the full and condensed electron configuration of barium (ba). 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Full electron configuration of barium: Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Enter the atomic number or symbol of.
Electron Configuration for Barium and Barium ion(Ba2+)
Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Calculate the full and condensed electron configuration of barium (ba). Full electron configuration of barium: Enter the atomic number or symbol of any element, including. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10.
How to Write the Electron Configuration for Barium (Ba)
Full electron configuration of barium: Enter the atomic number or symbol of any element, including. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Calculate the full and condensed electron configuration of barium (ba). Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328.
Calculate The Full And Condensed Electron Configuration Of Barium (Ba).
Barium is a chemical element of the periodic table with chemical symbol ba and atomic number 56 with an atomic weight of 137.328 u and is. Full electron configuration of barium: Enter the atomic number or symbol of any element, including. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2.