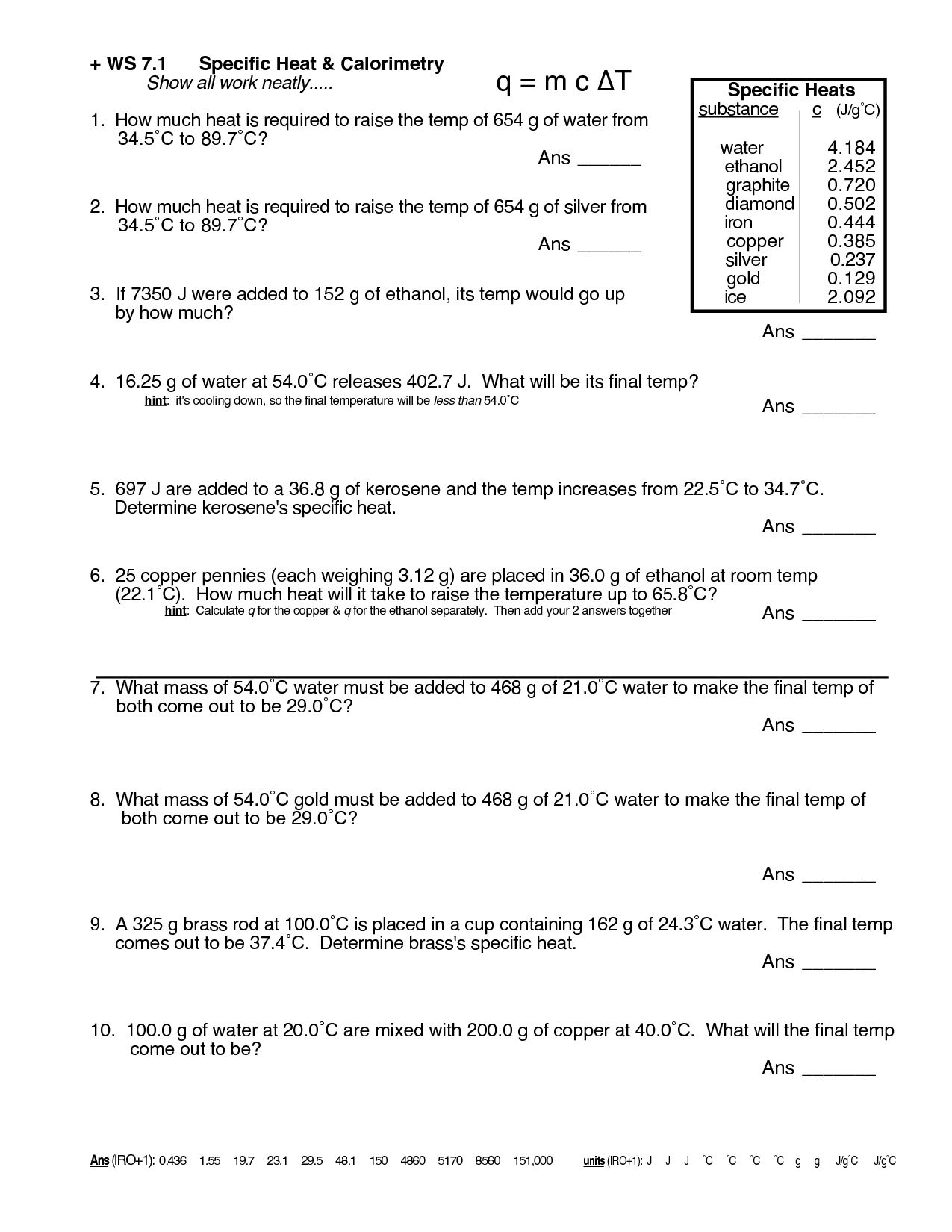
Calorimetry Worksheet Answers - Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. Calorimetry and specific heat calculation worksheet 1.
Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry and specific heat calculation worksheet 1. Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?
Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry and specific heat calculation worksheet 1. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter.
Calorimetry Worksheet Answer Key
Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry 1) if a gold ring with a mass of.
Calorimetry Worksheet Answer Key
Calorimetry and specific heat calculation worksheet 1. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that.
50 Calorimetry Worksheet Answer Key
Calorimetry and specific heat calculation worksheet 1. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Find the equilibrium temperature if 200.0 grams of cold.
50 Calorimetry Worksheet Answer Key
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry and specific heat calculation worksheet 1. Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry and molar.
Calorimetry Worksheet —
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry and specific heat calculation worksheet 1. How much energy is needed to change the temperature of 50.0 g of.
Heat And Calorimetry Worksheets
Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in..
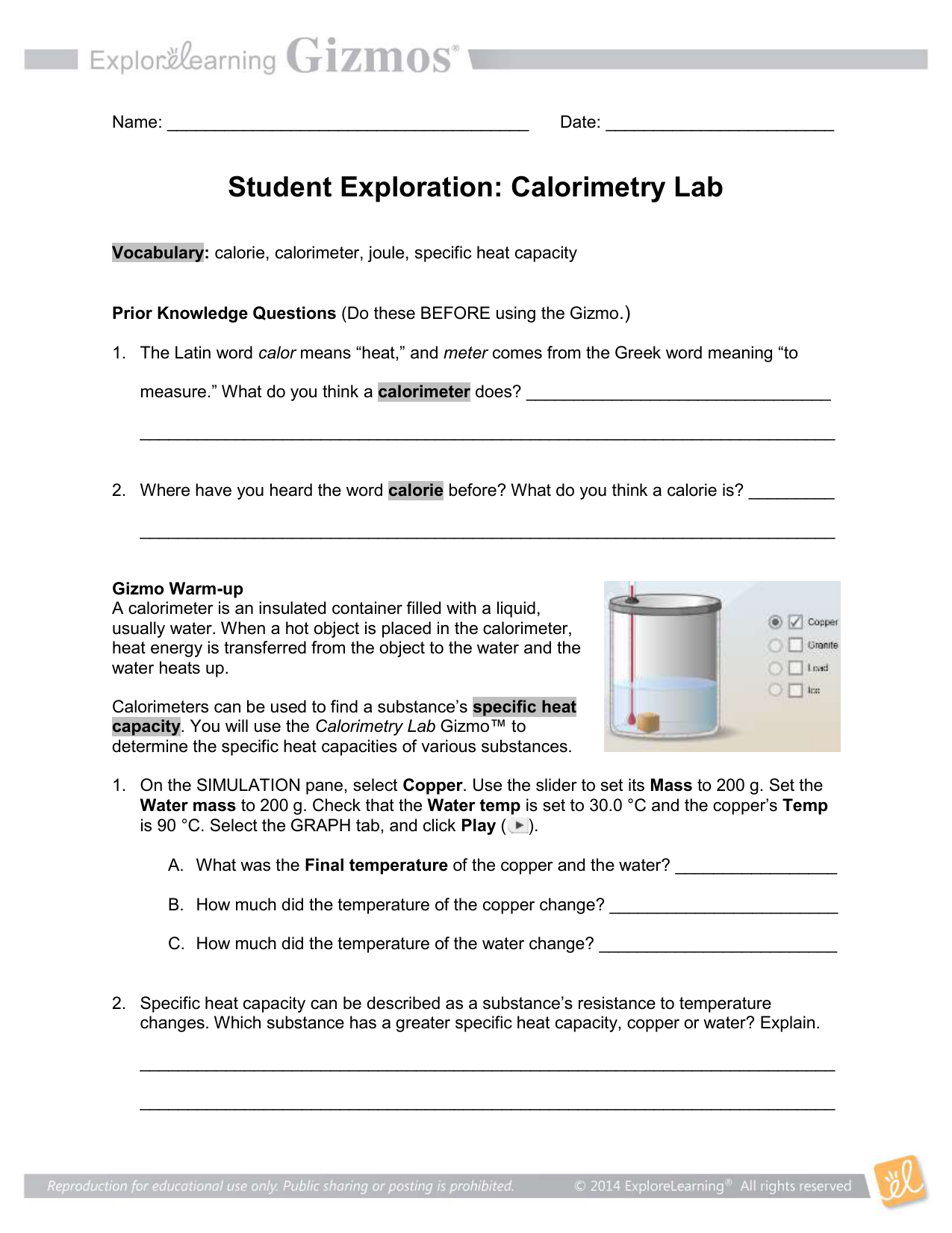
Copy of Copy of Calorimetry Lab SE Name Date Student Exploration
Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry and specific heat calculation worksheet 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c).
Chemistry Worksheet Heat And Calorimetry Problems Answers
Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry and specific heat calculation worksheet 1. How much energy is needed to change the temperature.
Calorimetry Worksheet Answer Key
Calorimetry and molar enthalpy worksheet answer key 1) 9.0 grams of charcoal (c) were completely consumed in a bomb calorimeter. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry and specific heat calculation worksheet 1. How much energy is needed to change the temperature of 50.0 g of.
Calorimetry Worksheet
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. Calorimetry and specific heat calculation worksheet 1. Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry 1) if.
Calorimetry 1) If A Gold Ring With A Mass Of 5.5 G Changes Temperature From 25.0 C To 28.0 C, How Much Energy (In.
How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Find the equilibrium temperature if 200.0 grams of cold water at 10.0oc is mixed. Calorimetry and specific heat calculation worksheet 1.