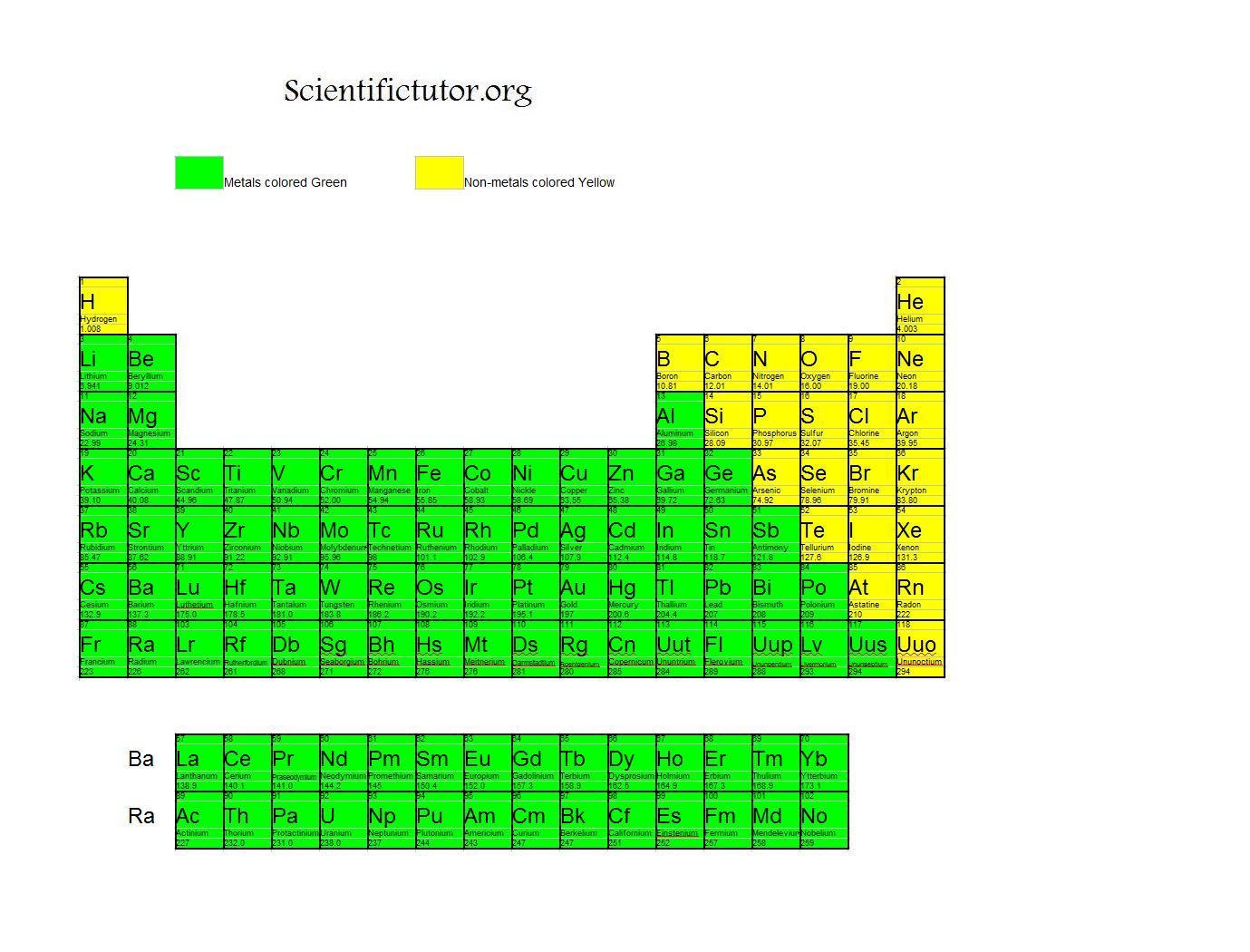
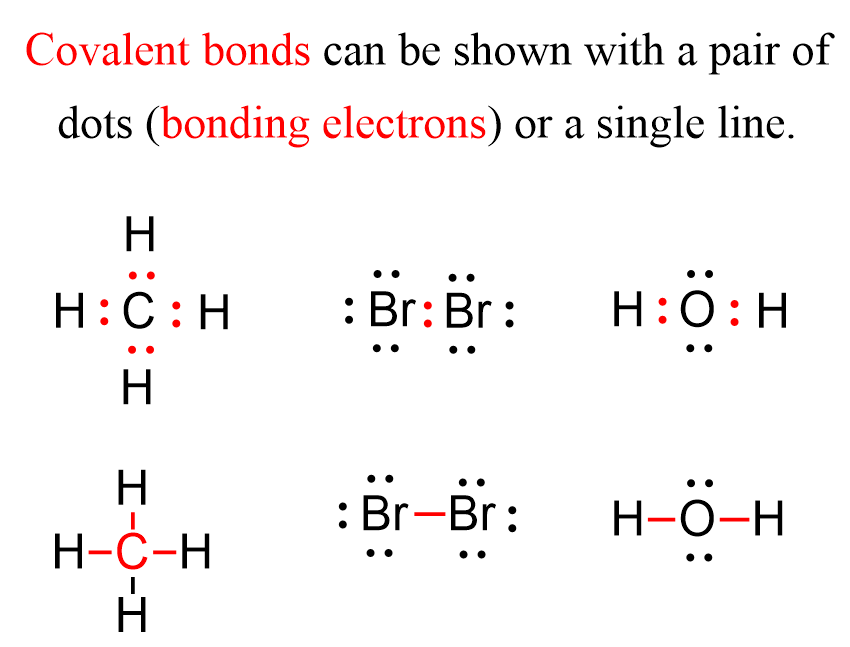
Can Metals Form Covalent Bonds - They usually form ionic bonds with nonmetals. However, there are exceptions, such as. Most of transition metal oxides and sulfates have. Metals typically do not form covalent bonds. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. It is very common in transition metal like platinum, palladium. However, it is not the way. There is no sharp line between ionic, metallic or covalent bonds. Metal do form covalent bond.
There is no sharp line between ionic, metallic or covalent bonds. Metals typically do not form covalent bonds. Metal do form covalent bond. They usually form ionic bonds with nonmetals. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Most of transition metal oxides and sulfates have. However, there are exceptions, such as. It is very common in transition metal like platinum, palladium. However, it is not the way.
My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Most of transition metal oxides and sulfates have. They usually form ionic bonds with nonmetals. Metal do form covalent bond. There is no sharp line between ionic, metallic or covalent bonds. However, there are exceptions, such as. However, it is not the way. It is very common in transition metal like platinum, palladium. Metals typically do not form covalent bonds.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Most of transition metal oxides and sulfates have. However, there are exceptions, such as. Metal do form covalent bond. There is no sharp line between ionic, metallic or covalent bonds. Metals typically do not form covalent bonds.
Chem Covalent, Ionic, and Metallic Bonds (Intramolecular Forces
However, there are exceptions, such as. Metals typically do not form covalent bonds. However, it is not the way. Most of transition metal oxides and sulfates have. It is very common in transition metal like platinum, palladium.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
They usually form ionic bonds with nonmetals. Metals typically do not form covalent bonds. There is no sharp line between ionic, metallic or covalent bonds. Metal do form covalent bond. However, there are exceptions, such as.
Reading Covalent Bonds Biology I
Metal do form covalent bond. Most of transition metal oxides and sulfates have. However, it is not the way. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Metals typically do not form covalent bonds.
Ionic, Covalent, and Metallic Bonds Differences and Similarities
There is no sharp line between ionic, metallic or covalent bonds. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Metal do form covalent bond. It is very common in transition metal like platinum, palladium. Metals typically do not form covalent bonds.
Covalent Bonds Pathways to Chemistry
Metal do form covalent bond. Most of transition metal oxides and sulfates have. However, there are exceptions, such as. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. They usually form ionic bonds with nonmetals.
Covalent Bond Definition, Types, and Examples
They usually form ionic bonds with nonmetals. It is very common in transition metal like platinum, palladium. Metal do form covalent bond. Most of transition metal oxides and sulfates have. Metals typically do not form covalent bonds.
Ionic, Covalent, and Metallic Bonds Differences and Similarities
However, there are exceptions, such as. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Metals typically do not form covalent bonds. They usually form ionic bonds with nonmetals. It is very common in transition metal like platinum, palladium.
Covalent bonding tecscience
My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Most of transition metal oxides and sulfates have. However, there are exceptions, such as. Metal do form covalent bond. It is very common in transition metal like platinum, palladium.
Covalent Bond Chemistry Steps
Metal do form covalent bond. Metals typically do not form covalent bonds. There is no sharp line between ionic, metallic or covalent bonds. However, there are exceptions, such as. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds.
It Is Very Common In Transition Metal Like Platinum, Palladium.
However, it is not the way. Metals typically do not form covalent bonds. However, there are exceptions, such as. My chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds.
Most Of Transition Metal Oxides And Sulfates Have.
They usually form ionic bonds with nonmetals. There is no sharp line between ionic, metallic or covalent bonds. Metal do form covalent bond.