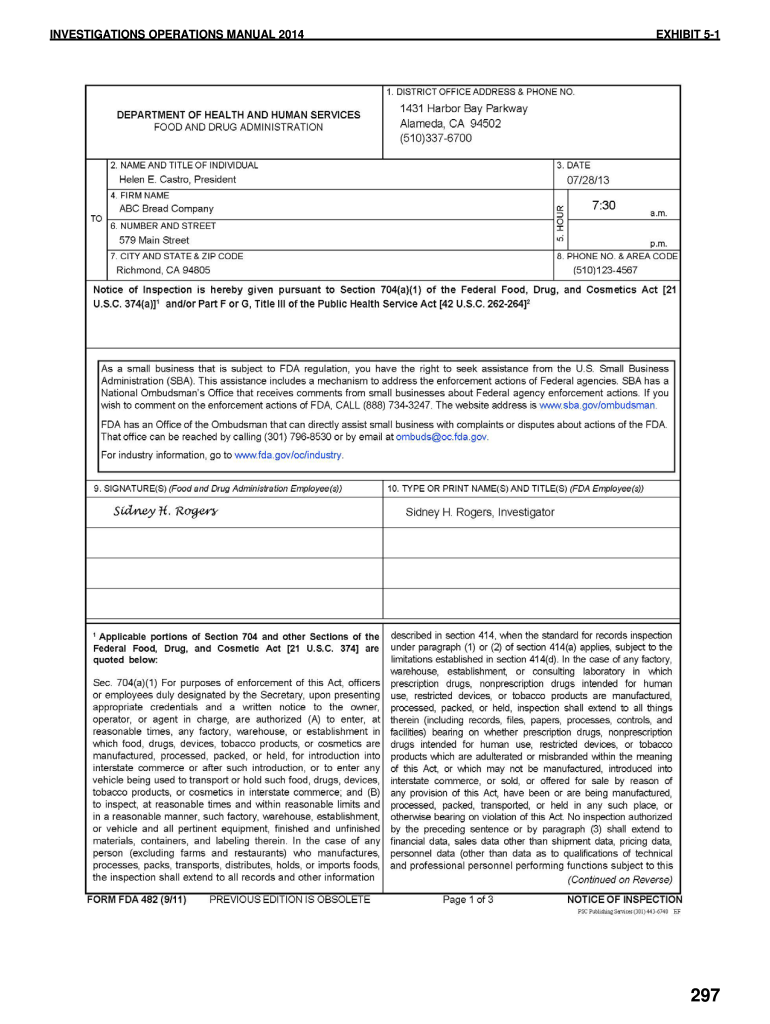
Fda Form 482 - I googled for fda forms 482, 483 and 484 but could not find it. Find out what they are testing for; In either case (oai or vai), within a few days to a week of the fda. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. If the investigator is saying that your facility is being. The 482 will give you the purpose of the inspection. Form 2892 now permits you to use one form for up to four product codes. Even on fda website, they have filled examples of these forms in.
If the investigator is saying that your facility is being. Even on fda website, they have filled examples of these forms in. Form 2892 now permits you to use one form for up to four product codes. The 482 will give you the purpose of the inspection. Find out what they are testing for; In either case (oai or vai), within a few days to a week of the fda. I googled for fda forms 482, 483 and 484 but could not find it. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not.
I googled for fda forms 482, 483 and 484 but could not find it. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. The 482 will give you the purpose of the inspection. Form 2892 now permits you to use one form for up to four product codes. Find out what they are testing for; Even on fda website, they have filled examples of these forms in. If the investigator is saying that your facility is being. In either case (oai or vai), within a few days to a week of the fda.
Form FDA 3542 Patent Information Submitted upon/after Approval of an
I googled for fda forms 482, 483 and 484 but could not find it. In either case (oai or vai), within a few days to a week of the fda. Even on fda website, they have filled examples of these forms in. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do.
Fda 2877 Form Fill Online, Printable, Fillable, Blank pdfFiller
The 482 will give you the purpose of the inspection. Find out what they are testing for; I googled for fda forms 482, 483 and 484 but could not find it. Even on fda website, they have filled examples of these forms in. If the investigator is saying that your facility is being.
Fda Form 2253 printable pdf download
The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. Even on fda website, they have filled examples of these forms in. The 482 will give you the purpose of the inspection. Find out what they are testing for; If the investigator is saying that your facility is being.
FDA Reports 58 Free Templates in PDF, Word, Excel Download
If the investigator is saying that your facility is being. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. I googled for fda forms 482, 483 and 484 but could not find it. Form 2892 now permits you to use one form for up to four product codes. Find out.
Form FDA 1571 Investigational New Drug Application Free Download
I googled for fda forms 482, 483 and 484 but could not find it. Even on fda website, they have filled examples of these forms in. The 482 will give you the purpose of the inspection. Form 2892 now permits you to use one form for up to four product codes. In either case (oai or vai), within a few.
FDA Applications 12 Free Templates in PDF, Word, Excel Download
Form 2892 now permits you to use one form for up to four product codes. If the investigator is saying that your facility is being. Find out what they are testing for; The 482 will give you the purpose of the inspection. I googled for fda forms 482, 483 and 484 but could not find it.
Form 482 Fill Online, Printable, Fillable, Blank pdfFiller
Even on fda website, they have filled examples of these forms in. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. In either case (oai or vai), within a few days to a week of the fda. Find out what they are testing for; If the investigator is saying that.
FDA forms inspection A Comprehensive Guide PharmaJia
I googled for fda forms 482, 483 and 484 but could not find it. The 482 will give you the purpose of the inspection. In either case (oai or vai), within a few days to a week of the fda. Even on fda website, they have filled examples of these forms in. Find out what they are testing for;
Form FDA 3613 Supplementary Information Certificate to Foreign
Form 2892 now permits you to use one form for up to four product codes. I googled for fda forms 482, 483 and 484 but could not find it. The 482 will give you the purpose of the inspection. Even on fda website, they have filled examples of these forms in. Find out what they are testing for;
20152020 Form PR 482.0 Fill Online, Printable, Fillable, Blank pdfFiller
Even on fda website, they have filled examples of these forms in. If the investigator is saying that your facility is being. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. In either case (oai or vai), within a few days to a week of the fda. Find out what.
Form 2892 Now Permits You To Use One Form For Up To Four Product Codes.
If the investigator is saying that your facility is being. The warning letter notes a timeframe to reply to fda, but the other letters (vai or nai) do not. Find out what they are testing for; I googled for fda forms 482, 483 and 484 but could not find it.
Even On Fda Website, They Have Filled Examples Of These Forms In.
The 482 will give you the purpose of the inspection. In either case (oai or vai), within a few days to a week of the fda.