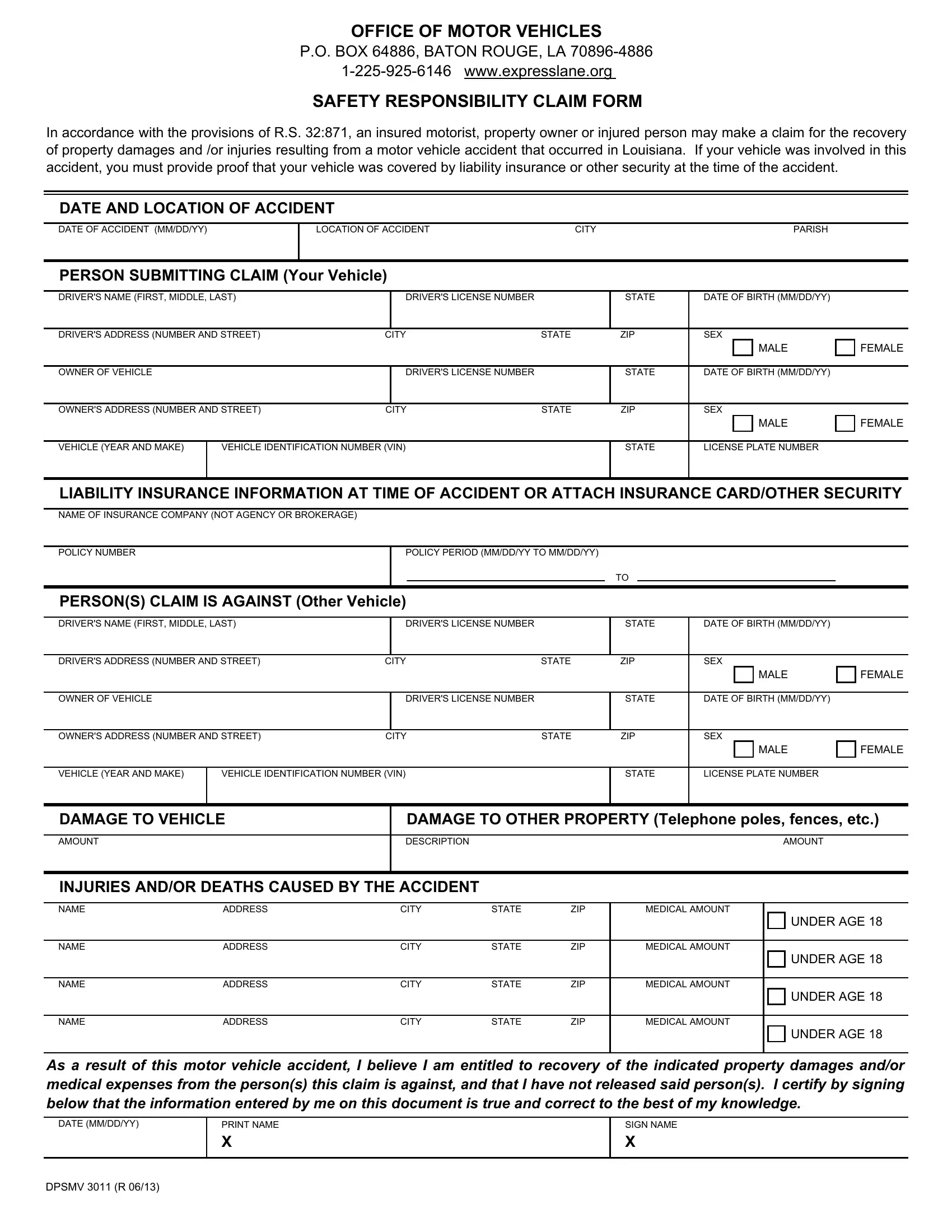
Form 3011 Health Canada - Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Hc3011:drug submission application form for: Find out where to send the form,. Human, veterinary or disinfectant drugs and clinical. Health canada requires the personal information to process regulatory application forms related to human and. Human, veterinary or disinfectant drugs and clinical trial application/attestation author:. Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic and. Drug submission application form for: On the canadian 3011 form please see health canada’s website for details including information and instructions on filing.
Drug submission application form for: Human, veterinary or disinfectant drugs and clinical trial application/attestation author:. Find out where to send the form,. (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Health canada requires the personal information to process regulatory application forms related to human and. Human, veterinary or disinfectant drugs and clinical. On the canadian 3011 form please see health canada’s website for details including information and instructions on filing. Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic and. Hc3011:drug submission application form for:
Health canada requires the personal information to process regulatory application forms related to human and. Human, veterinary or disinfectant drugs and clinical trial application/attestation author:. Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic and. Human, veterinary or disinfectant drugs and clinical. Drug submission application form for: (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Hc3011:drug submission application form for: Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Find out where to send the form,. On the canadian 3011 form please see health canada’s website for details including information and instructions on filing.
Cpp application form printable Fill out & sign online DocHub
Hc3011:drug submission application form for: Human, veterinary or disinfectant drugs and clinical. Health canada requires the personal information to process regulatory application forms related to human and. Drug submission application form for: Find out where to send the form,.
ADM043011correctionamendmentto PersonalHealthInformationform
Human, veterinary or disinfectant drugs and clinical. Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic and. (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Health canada requires the personal information to process regulatory application forms.
202107 A2021000066 Health Canada DocumentCloud
(2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Human, veterinary or disinfectant drugs and clinical trial application/attestation author:. Human, veterinary or disinfectant drugs and clinical. Please note that, as of.
Form Dpsmv 3011 ≡ Fill Out Printable PDF Forms Online
Human, veterinary or disinfectant drugs and clinical trial application/attestation author:. Find out where to send the form,. On the canadian 3011 form please see health canada’s website for details including information and instructions on filing. Health canada requires the personal information to process regulatory application forms related to human and. Please note that, as of october 1, 2022, the 3011.
Health Canada Public Health Agency Canada Guidelines Public Engagement
Hc3011:drug submission application form for: (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Human, veterinary or disinfectant drugs and clinical trial application/attestation author:. Drug submission application form for: Find out where to send the form,.
Gov handbook Fill out & sign online DocHub
Hc3011:drug submission application form for: Drug submission application form for: (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Health canada requires the personal information to process regulatory application forms related.
Ohio Form 3011 ≡ Fill Out Printable PDF Forms Online
Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Drug submission application form for: Find out where to send the form,. On the canadian 3011 form please see health canada’s website for details including information and instructions on filing. Human, veterinary or disinfectant drugs and clinical.
202105 A2020001798 Health Canada DocumentCloud
Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic and. On the canadian 3011 form please see health canada’s website for details including information and instructions on filing. Hc3011:drug submission application form for: Health canada requires the personal information to process regulatory application forms related to human and. Drug.
Health Canada Form HCSC 3011 Drug Submission Application
Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Drug submission application form for: On the canadian 3011 form please see health canada’s website for details including information and instructions on filing. Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic.
BA3011251 Flat Adapter for BA3011 Elton Manufacturing
Drug submission application form for: Health canada requires the personal information to process regulatory application forms related to human and. Human, veterinary or disinfectant drugs and clinical. Find out where to send the form,. (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada.
Health Canada Requires The Personal Information To Process Regulatory Application Forms Related To Human And.
Find out where to send the form,. Human, veterinary or disinfectant drugs and clinical. (2 days ago) learn how to fill out the hc/sc 3011 form for different types of drug submissions to health canada. Drug submission application form for:
Human, Veterinary Or Disinfectant Drugs And Clinical Trial Application/Attestation Author:.
On the canadian 3011 form please see health canada’s website for details including information and instructions on filing. Please note that, as of october 1, 2022, the 3011 should no longer be used for applications for pharmaceutical, biologic and. Health canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. Hc3011:drug submission application form for: