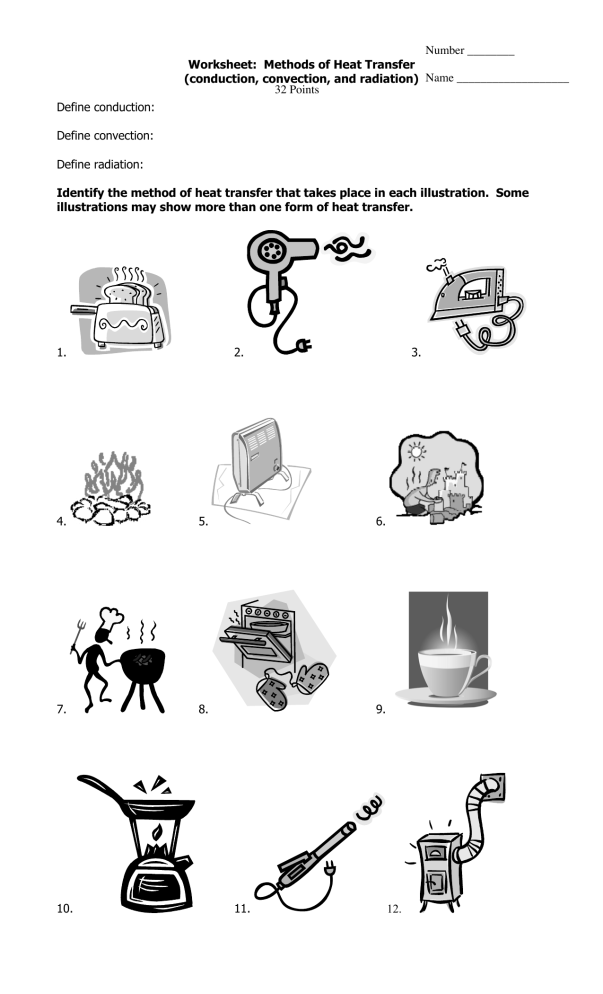
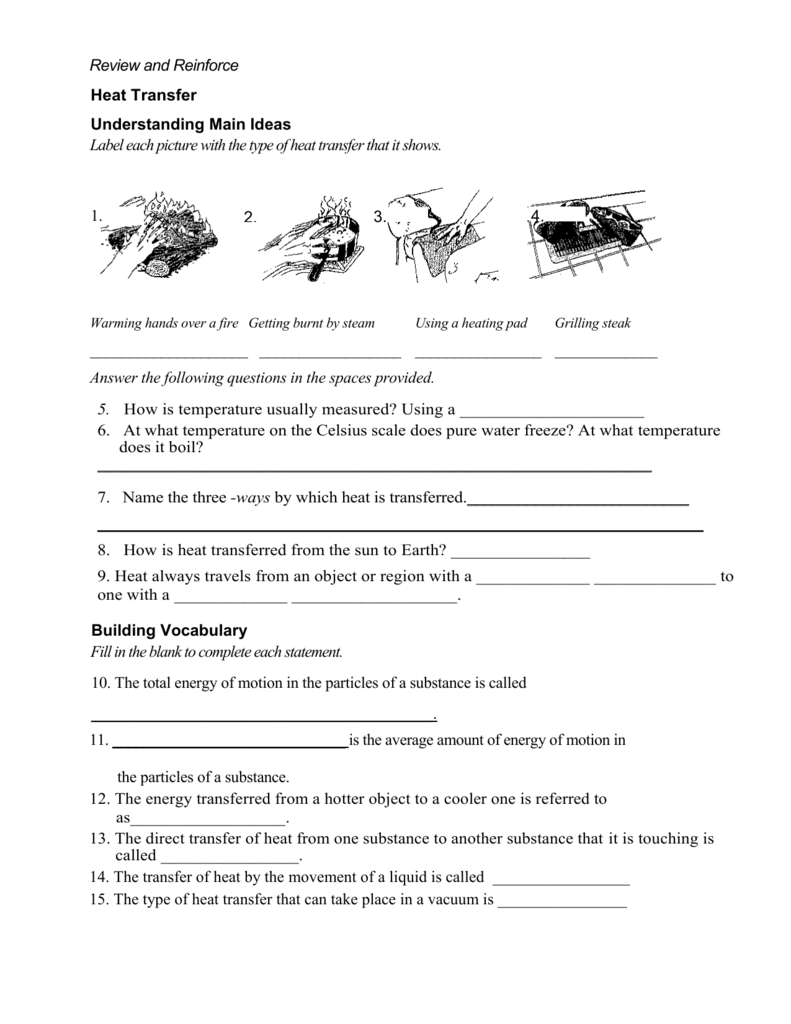
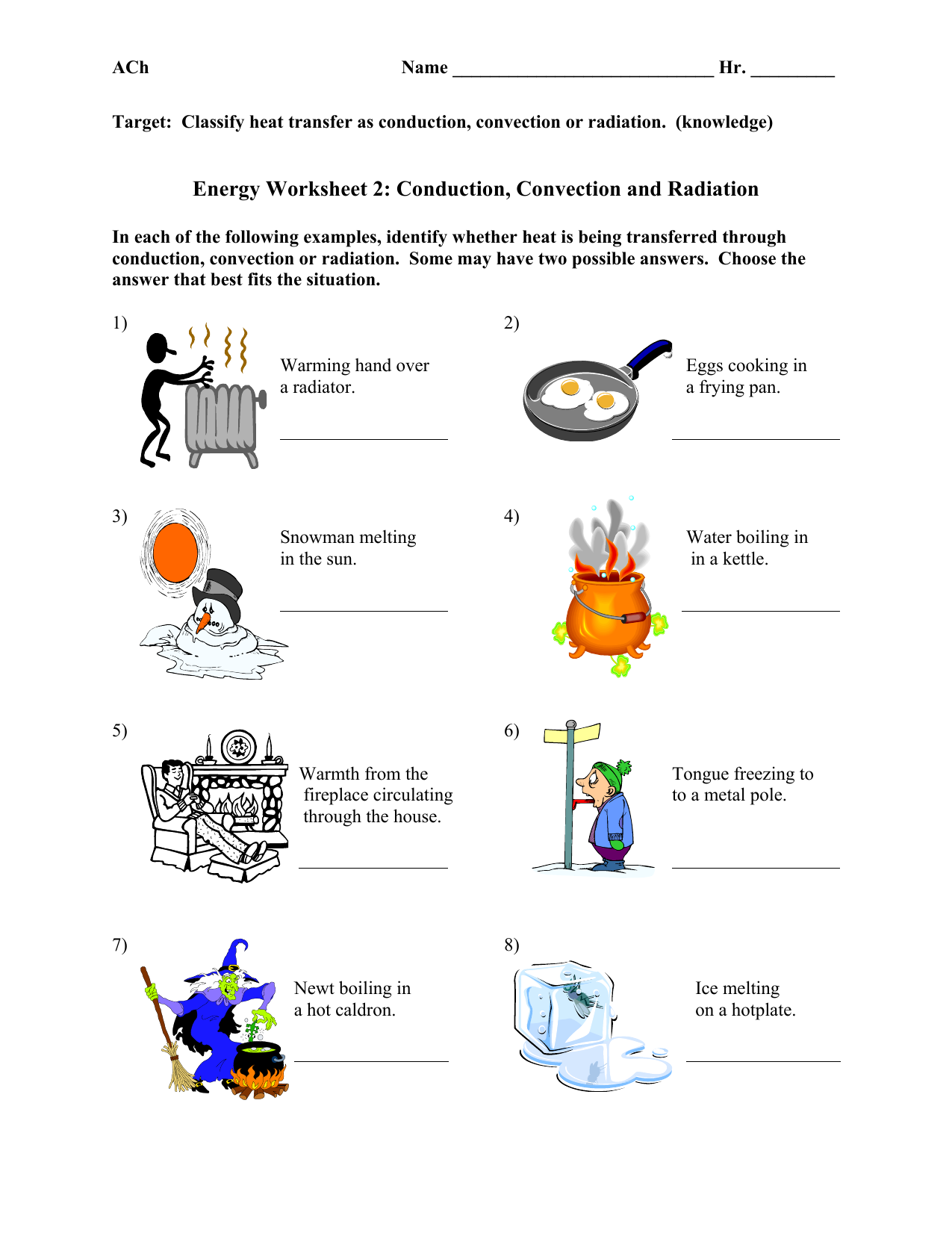
Heat And Heat Transfer Worksheet - Heat is transferred in three ways: Q = m*c*δt where q is heat (kj), m is the mass of. The direct transfer of heat. Heat transfer specific heat capacity 1. Radiation is the direct transfer of energy. (b) find the specific heat. In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c?
(b) find the specific heat. (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? The direct transfer of heat. In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). Heat transfer specific heat capacity 1. Q = m*c*δt where q is heat (kj), m is the mass of. Radiation is the direct transfer of energy. Heat is transferred in three ways:
Heat is transferred in three ways: (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? Radiation is the direct transfer of energy. Q = m*c*δt where q is heat (kj), m is the mass of. (b) find the specific heat. In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). The direct transfer of heat. Heat transfer specific heat capacity 1.
Heat Transfer
Q = m*c*δt where q is heat (kj), m is the mass of. Heat transfer specific heat capacity 1. In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). Radiation is the direct transfer of energy. The direct transfer of heat.
Worksheet Methods Of Heat Transfer
Radiation is the direct transfer of energy. The direct transfer of heat. Heat transfer specific heat capacity 1. Q = m*c*δt where q is heat (kj), m is the mass of. Heat is transferred in three ways:
Heat Transfer interactive exercise for 7 Live Worksheets Worksheets
(b) find the specific heat. Radiation is the direct transfer of energy. In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? Heat is transferred in three ways:
the heat transfer worksheet for students to practice heat transfer in
Q = m*c*δt where q is heat (kj), m is the mass of. Heat transfer specific heat capacity 1. Heat is transferred in three ways: Radiation is the direct transfer of energy. (b) find the specific heat.
30 Methods Of Heat Transfer Worksheet Education Template
Heat transfer specific heat capacity 1. The direct transfer of heat. Heat is transferred in three ways: (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation).
Heat Transfer Worksheet Answers
Q = m*c*δt where q is heat (kj), m is the mass of. Heat transfer specific heat capacity 1. (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? Heat is transferred in three ways: (b) find the specific heat.
Heat Transfer Worksheet Answers
Heat transfer specific heat capacity 1. Radiation is the direct transfer of energy. Q = m*c*δt where q is heat (kj), m is the mass of. (b) find the specific heat. The direct transfer of heat.
Heat And Heat Transfer Worksheets
Heat is transferred in three ways: (b) find the specific heat. Heat transfer specific heat capacity 1. (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? Radiation is the direct transfer of energy.
SOLUTION Methods of Heat Transfer Worksheet Studypool
The direct transfer of heat. Q = m*c*δt where q is heat (kj), m is the mass of. In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). Radiation is the direct transfer of energy. (b) find the specific heat.
The Direct Transfer Of Heat.
In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). Heat is transferred in three ways: (a) how much energy is lost when 50 g of iron cools from 45°c to 15°c? Radiation is the direct transfer of energy.
Heat Transfer Specific Heat Capacity 1.
(b) find the specific heat. Q = m*c*δt where q is heat (kj), m is the mass of.