How Many Bonds Can Water Form - Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. The oxygen atom has a slightly negative. This occurs as the oxygen atom can. Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. Each water molecule can form up to four hydrogen bonds.
Each water molecule can form up to four hydrogen bonds. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. The oxygen atom has a slightly negative. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. This occurs as the oxygen atom can. Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections.
A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. The oxygen atom has a slightly negative. Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. Each water molecule can form up to four hydrogen bonds. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. This occurs as the oxygen atom can. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule.
Types of Chemical Bonds
A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. This occurs as the oxygen.
What is the name and type of bonds present in water Science
Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. This occurs as the oxygen atom can. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. The oxygen atom has.
Water Definition, Chemical Formula, Structure, Molecule, & Facts
Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. The oxygen atom has a slightly negative. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. This occurs as the oxygen atom can. Each water molecule can form hydrogen bonds with.
Hydrogen Bonds — Overview & Examples Expii
Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Each water molecule can form up to four hydrogen bonds. The oxygen atom has a slightly negative. This occurs as the oxygen atom can.
Are Hydrogen Bonds The Strongest Interactions Between Molecules?
This occurs as the oxygen atom can. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. The oxygen atom has a slightly negative. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Each water molecule can form hydrogen bonds with.
Are Hydrogen Bonds The Strongest Interactions Between Molecules?
Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. The oxygen atom has a slightly negative. This occurs as the oxygen atom can. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. Each water molecule can form up to four.
Reading Covalent Bonds Biology (Early Release)
This occurs as the oxygen atom can. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. The oxygen atom has.
Primary and Secondary Bonds Owlcation
Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. A water molecule (h2o) consists of two hydrogen atoms bonded to a single oxygen atom. The oxygen atom has a slightly negative. Each water molecule can form up to four hydrogen bonds. Water molecules can form intramolecular hydrogen bonds, which are bonds.
Water Molecule Diagram Bonds
This occurs as the oxygen atom can. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. Each water molecule can form up to four hydrogen bonds. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. A water molecule (h2o) consists.
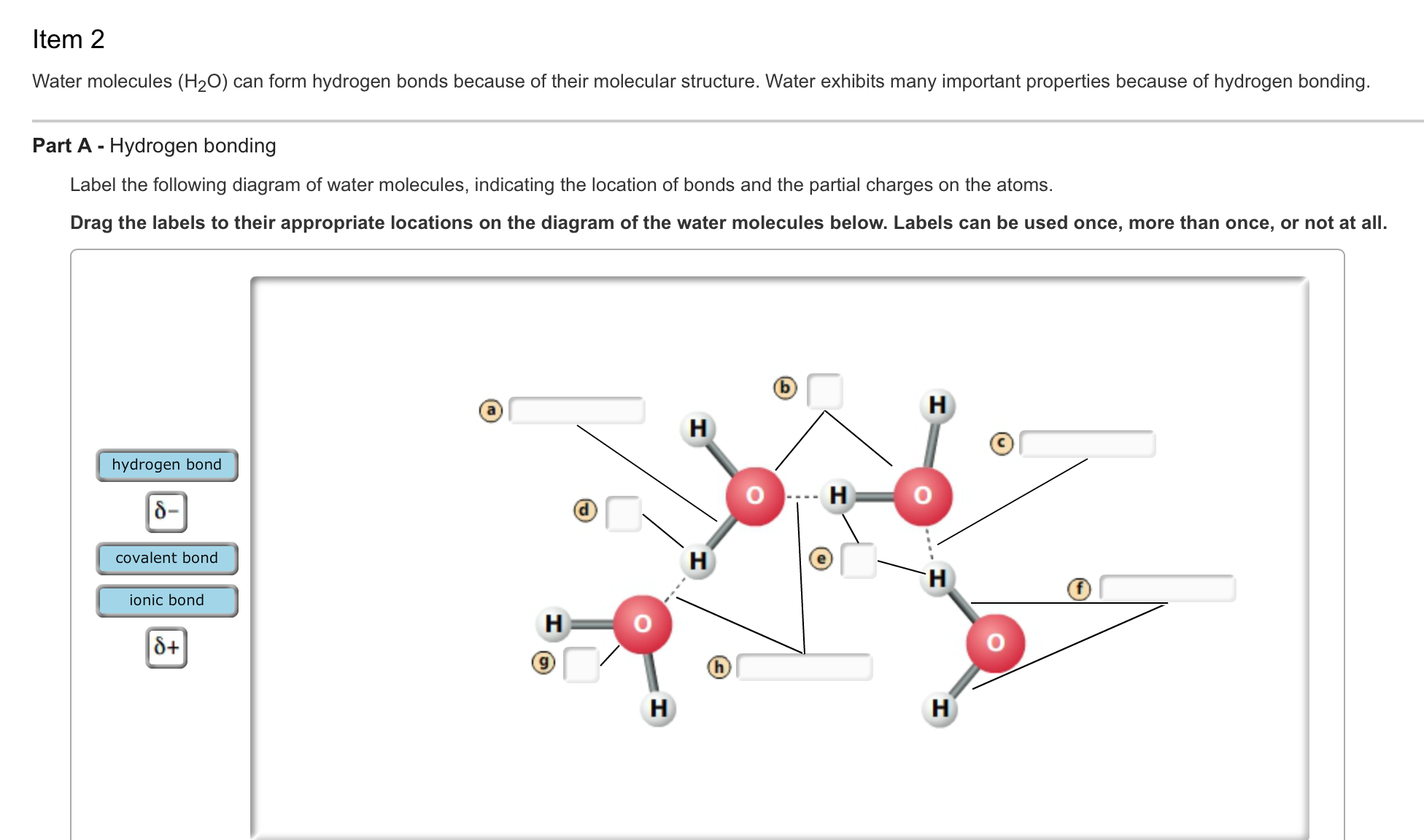
Solved Water molecules (H_2O) can form hydrogen bonds
Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. This occurs as the oxygen atom can. The oxygen atom has a slightly negative. Water molecules can form intramolecular hydrogen bonds, which are bonds between atoms within the same molecule. A water molecule (h2o) consists of two hydrogen atoms bonded to a.
A Water Molecule (H2O) Consists Of Two Hydrogen Atoms Bonded To A Single Oxygen Atom.
Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. The oxygen atom has a slightly negative. Each water molecule can form hydrogen bonds with up to three others, constantly shifting and reforming these connections. Each water molecule can form up to four hydrogen bonds.
Water Molecules Can Form Intramolecular Hydrogen Bonds, Which Are Bonds Between Atoms Within The Same Molecule.
This occurs as the oxygen atom can.