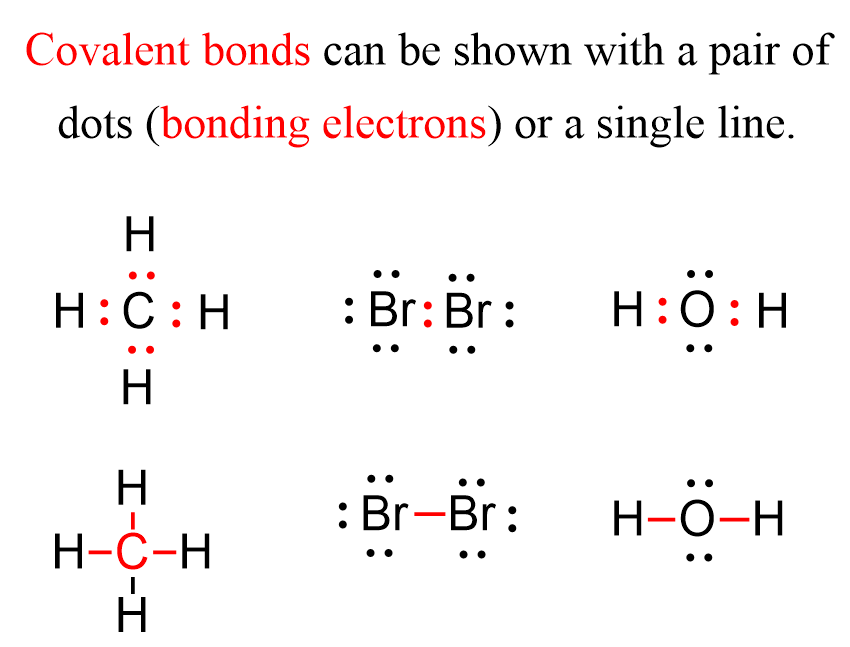
How Many Covalent Bonds Can Sulfur Form - Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. How many covalent bonds are formed? Sulfur can form two covalent bonds with other atoms. The number of bonds that an atom can form can often be predicted from the number of electrons. This is because sulfur has six valence electrons, which are the electrons. Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3).
The number of bonds that an atom can form can often be predicted from the number of electrons. This is because sulfur has six valence electrons, which are the electrons. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). Sulfur can form two covalent bonds with other atoms. How many covalent bonds are formed? Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons.
Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). Sulfur can form two covalent bonds with other atoms. This is because sulfur has six valence electrons, which are the electrons. Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. How many covalent bonds are formed? The number of bonds that an atom can form can often be predicted from the number of electrons. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell.
Why can sulfur bond 6 times?
This is because sulfur has six valence electrons, which are the electrons. The number of bonds that an atom can form can often be predicted from the number of electrons. Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. How many covalent bonds are formed? Now sulfur has 6 unpaired electrons which.
PPT COVALENT BONDING PowerPoint Presentation, free download ID1988932
The number of bonds that an atom can form can often be predicted from the number of electrons. Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. Sulfur can form two covalent bonds with other atoms. Now sulfur.
Why Can Sulfur Make 6 Bonds? Top 11 Best Answers
Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). How many covalent bonds are formed? Sulfur, in the most common circumstances, can form two covalent bonds due to having six.
sulfur Definition, Element, Symbol, Uses, & Facts Britannica
Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. How many covalent bonds are formed? Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). The number of bonds that an atom can form can often be predicted from the number of electrons. Now sulfur has 6 unpaired.
Covalent Bond Chemistry Steps
The number of bonds that an atom can form can often be predicted from the number of electrons. This is because sulfur has six valence electrons, which are the electrons. Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. Sulfur can form two covalent bonds with other atoms. How many covalent bonds.
How Many Bonds Does Nitrogen Form Asking List
Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. How many covalent bonds are formed? Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). The number of bonds that an atom can form can often be predicted from the.
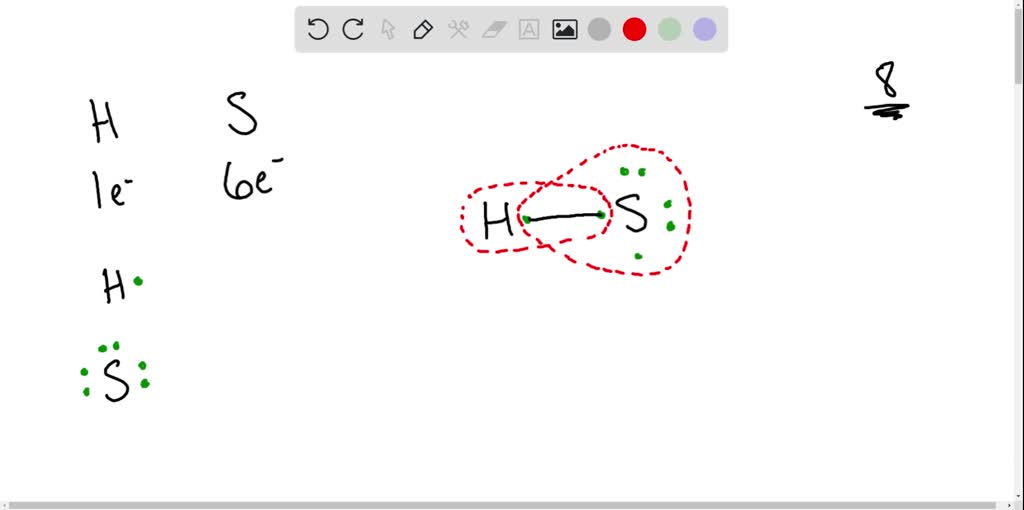
SOLVEDThe atomic number of sulfur (S) is 16. Sulfur combines with
Sulfur can form two covalent bonds with other atoms. This is because sulfur has six valence electrons, which are the electrons. Sulfur can form three covalent bonds with elements like carbon (ch3sch3) and phosphorus (ps3). Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. The number of bonds that an atom can.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The number of bonds that an atom can form can often be predicted from the number of electrons. This is because sulfur has six valence electrons, which are the electrons. Sulfur can form two covalent bonds with other atoms. How many covalent bonds are formed? Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to.
How Many Bonds Does Nitrogen Form Asking List
Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. The number of bonds that an atom can form can often be predicted from the number of electrons..
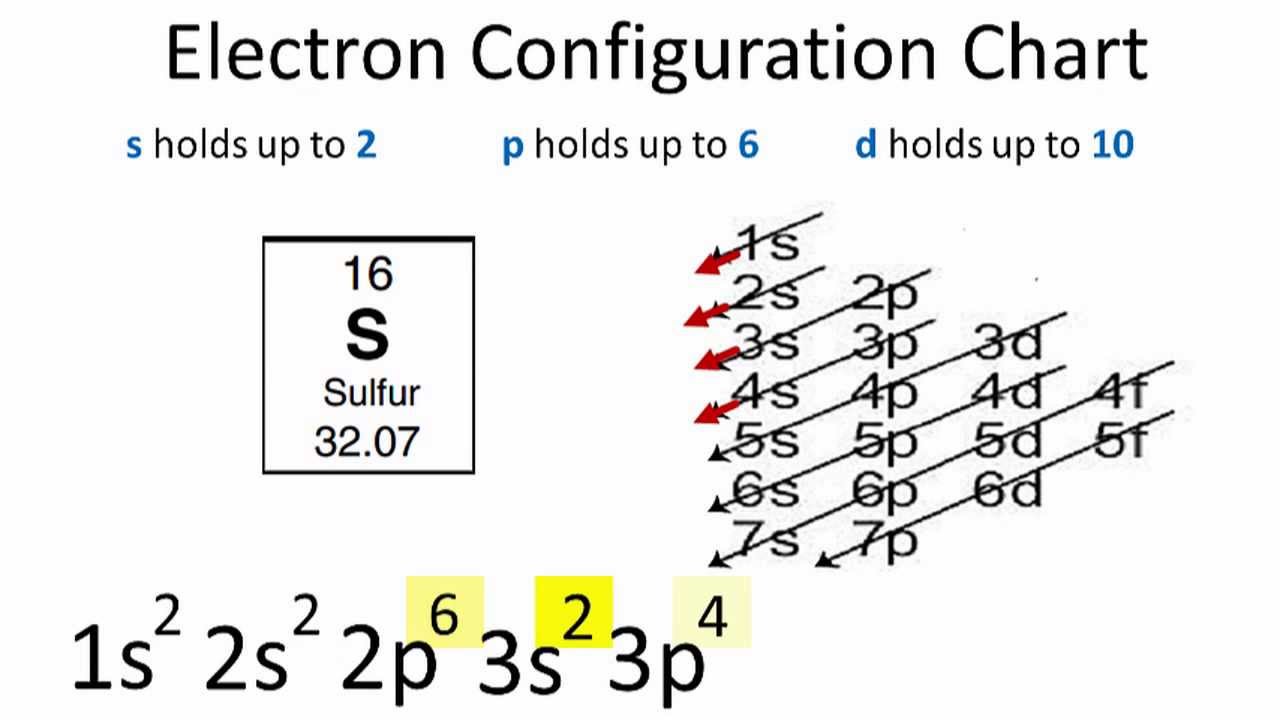
Sulfur Electron Configuration (S) with Orbital Diagram
Sulfur can form two covalent bonds with other atoms. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. How many covalent bonds are formed? This is because sulfur has six valence electrons, which are the electrons. Sulfur, in the most common circumstances, can form.
Sulfur Can Form Three Covalent Bonds With Elements Like Carbon (Ch3Sch3) And Phosphorus (Ps3).
How many covalent bonds are formed? Sulfur, in the most common circumstances, can form two covalent bonds due to having six valence electrons. The number of bonds that an atom can form can often be predicted from the number of electrons. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell.
Sulfur Can Form Two Covalent Bonds With Other Atoms.
This is because sulfur has six valence electrons, which are the electrons.