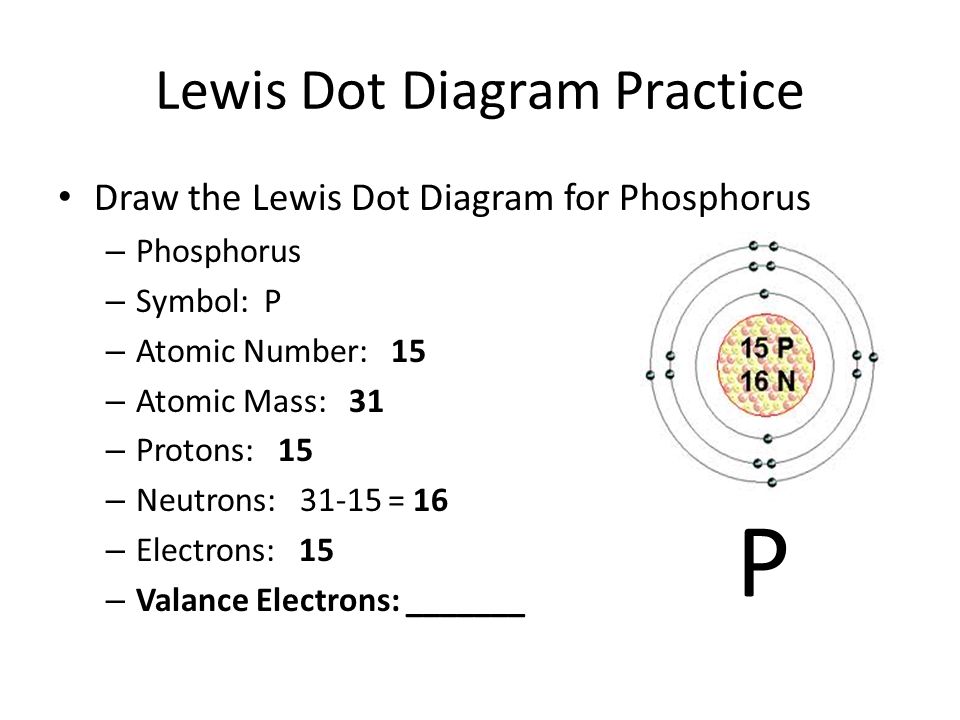
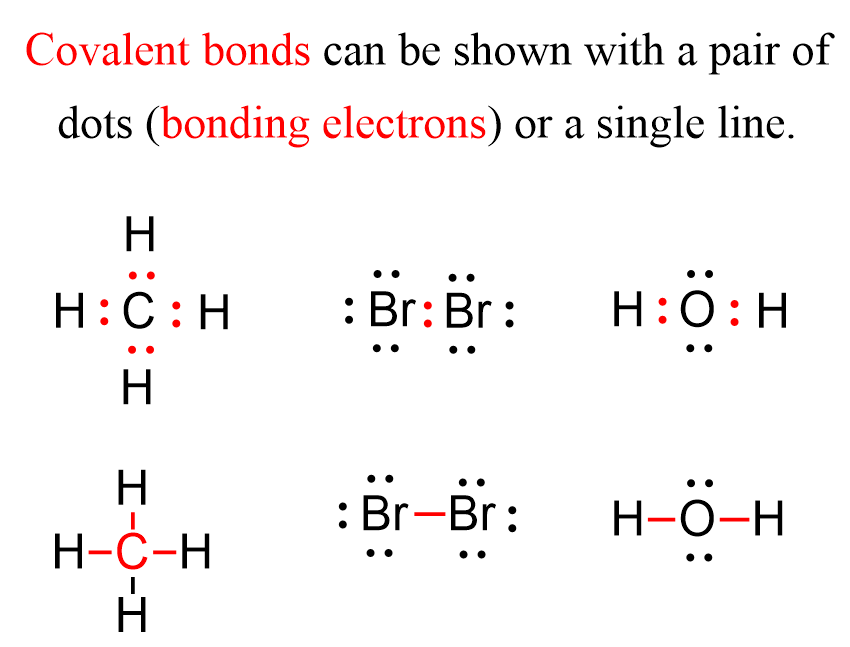
How Many Covalent Bonds Does Phosphorus Form - This bonding pattern is exhibited in compounds such. These bonds can be single,. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. The answer to the question how many bonds can phosphorus form? is straightforward: A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Phosphorus can form up to 5. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms.
This bonding pattern is exhibited in compounds such. The answer to the question how many bonds can phosphorus form? is straightforward: These bonds can be single,. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Phosphorus can form up to 5. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms.
Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. This bonding pattern is exhibited in compounds such. The answer to the question how many bonds can phosphorus form? is straightforward: Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. These bonds can be single,. Phosphorus can form up to 5.
Covalent bonding tecscience
Phosphorus can form up to 5. These bonds can be single,. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. This bonding pattern is exhibited in compounds such.
SOLVED how many covalent bonds does carbon form if each of its
Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. This bonding pattern is exhibited in compounds such. These bonds can be single,. Phosphorus can form up to 5. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms.
How Many Bonds Does Nitrogen Form Asking List
Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. The answer to the question how many bonds can phosphorus form? is straightforward: These bonds can.
Triple Covalent Bond Definition and Examples
Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. These bonds can be single,. The answer to the question how many bonds can phosphorus form? is straightforward: Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5),.
How Many Protons Does Phosphorus Have Asking List
The answer to the question how many bonds can phosphorus form? is straightforward: A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. This bonding pattern is exhibited in compounds such. These bonds can be single,.
Covalent Bond Chemistry Steps
This bonding pattern is exhibited in compounds such. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. These bonds can be single,. A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Phosphorus can form up to 5.
Reading Covalent Bonds Biology I
Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. The answer to the question how many bonds can phosphorus form? is straightforward: These bonds can be single,. Phosphorus can form up to 5. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Phosphorus can form up to 5. These bonds can be single,. The answer to the question how many bonds can phosphorus form? is straightforward: Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds.
How does phosphorus form 5 covalent bonds? The Unconditional Guru
The answer to the question how many bonds can phosphorus form? is straightforward: A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds. Phosphorus can form up to 5. These bonds can be single,.
[Solved] PCl3F2 How many single bonds does phosphorus have it? Course
These bonds can be single,. This bonding pattern is exhibited in compounds such. The answer to the question how many bonds can phosphorus form? is straightforward: Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms. Based on its electron configuration, phosphorus can form a maximum of three covalent bonds.
This Bonding Pattern Is Exhibited In Compounds Such.
These bonds can be single,. The answer to the question how many bonds can phosphorus form? is straightforward: A phosphorus atom can form a maximum of five covalent bonds as seen in phosphorus pentachloride (pcl5), where it. Phosphorus (v) typically forms four bonds, most commonly with oxygen atoms.
Based On Its Electron Configuration, Phosphorus Can Form A Maximum Of Three Covalent Bonds.
Phosphorus can form up to 5.