How To Calculate Root Mean Square Velocity - This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. The root mean square velocity (rms velocity) is a way to find a single velocity value for the particles. Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. The average velocity of gas particles is found using the root mean square velocity formula: Why is root mean square velocity important in thermodynamics and. Woosh, your result is instantly computed! Our root mean square speed calculator gives you an effortless way to calculate the rms speed for an ideal and mostly monoatomic gases. Vᵣₘₛ = √(3 * r * t / m), where r is the gas constant, t is the temperature, and m is the molar mass. Μ rms = (3rt/m) ½ To calculate, we need to:
Μ rms = (3rt/m) ½ Woosh, your result is instantly computed! To calculate, we need to: Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. How do i calculate root mean square velocity? This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. Why is root mean square velocity important in thermodynamics and. The root mean square velocity (rms velocity) is a way to find a single velocity value for the particles. The average velocity of gas particles is found using the root mean square velocity formula: Our root mean square speed calculator gives you an effortless way to calculate the rms speed for an ideal and mostly monoatomic gases.
Why is root mean square velocity important in thermodynamics and. How do i calculate root mean square velocity? Woosh, your result is instantly computed! Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. The root mean square velocity (rms velocity) is a way to find a single velocity value for the particles. To calculate, we need to: The average velocity of gas particles is found using the root mean square velocity formula: Μ rms = (3rt/m) ½ Vᵣₘₛ = √(3 * r * t / m), where r is the gas constant, t is the temperature, and m is the molar mass.
Fine Beautiful Root Mean Square Velocity Derivation Pdf What Is
Woosh, your result is instantly computed! Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. How do i calculate root mean square velocity? The root mean square velocity (rms velocity) is a way to find a single velocity value for the particles. The average velocity.
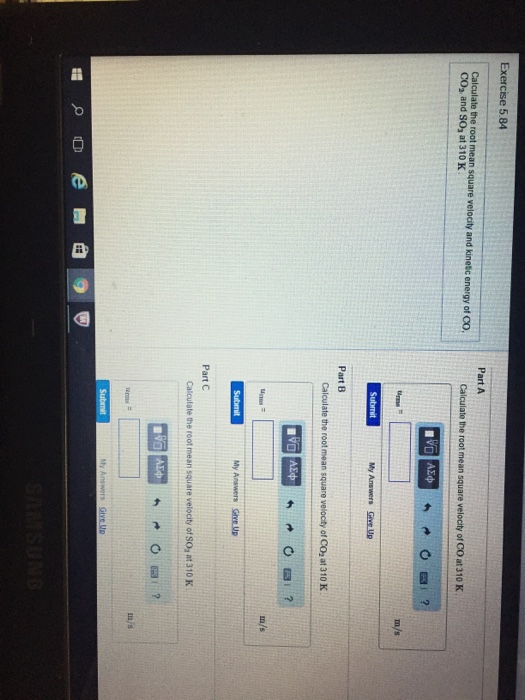
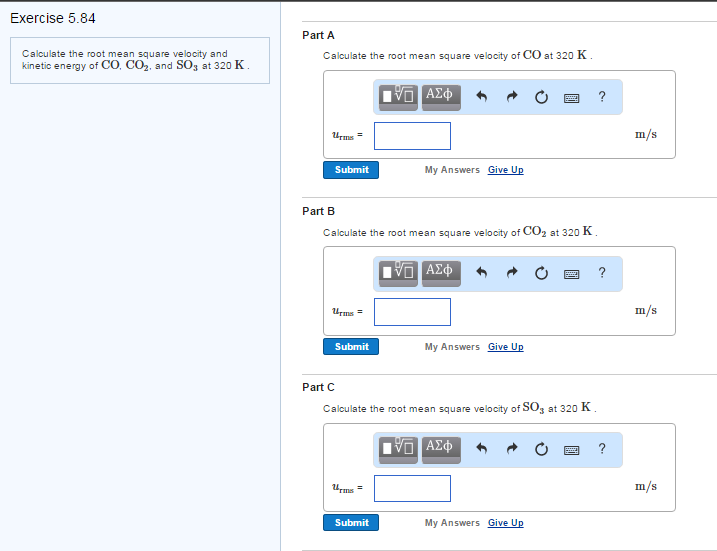
Solved Calculate the root mean square velocity and
The average velocity of gas particles is found using the root mean square velocity formula: Woosh, your result is instantly computed! Our root mean square speed calculator gives you an effortless way to calculate the rms speed for an ideal and mostly monoatomic gases. Why is root mean square velocity important in thermodynamics and. This online chemistry calculator may be.
Fine Beautiful Root Mean Square Velocity Derivation Pdf What Is
Μ rms = (3rt/m) ½ The average velocity of gas particles is found using the root mean square velocity formula: This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. Vᵣₘₛ = √(3 * r * t / m), where r is the gas constant, t is the temperature,.
Root Mean Square Velocity Calculator
To calculate, we need to: Μ rms = (3rt/m) ½ This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. Vᵣₘₛ = √(3 * r * t / m), where r is the gas constant, t is the temperature, and m is the molar mass. Use the root mean.
Calculate (a) The mean velocity, (b) The root mean square velocity and th..
Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. Our root mean square speed calculator gives you an effortless way to calculate the rms.
SOLVEDCalculate The Root Mean Square Velocities Of CH4(g), 44 OFF
Μ rms = (3rt/m) ½ How do i calculate root mean square velocity? This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. Why is root mean square velocity important in thermodynamics and. To calculate, we need to:
Free (RMS) Root mean Square velocity Animation by Joana Barreira
How do i calculate root mean square velocity? Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. Woosh, your result is instantly computed! To calculate, we need to: This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average.
Solved Calculate the root mean square velocity and
Why is root mean square velocity important in thermodynamics and. The average velocity of gas particles is found using the root mean square velocity formula: Our root mean square speed calculator gives you an effortless way to calculate the rms speed for an ideal and mostly monoatomic gases. This online chemistry calculator may be used to calculate root mean square.
34+ Root Mean Square Velocity Calculator OlufemiSalymat
Woosh, your result is instantly computed! Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. To calculate, we need to: The average velocity of gas particles is found using the root mean square velocity formula: Our root mean square speed calculator gives you an effortless.
Fine Beautiful Root Mean Square Velocity Derivation Pdf What Is
Μ rms = (3rt/m) ½ Our root mean square speed calculator gives you an effortless way to calculate the rms speed for an ideal and mostly monoatomic gases. Why is root mean square velocity important in thermodynamics and. The average velocity of gas particles is found using the root mean square velocity formula: Vᵣₘₛ = √(3 * r * t.
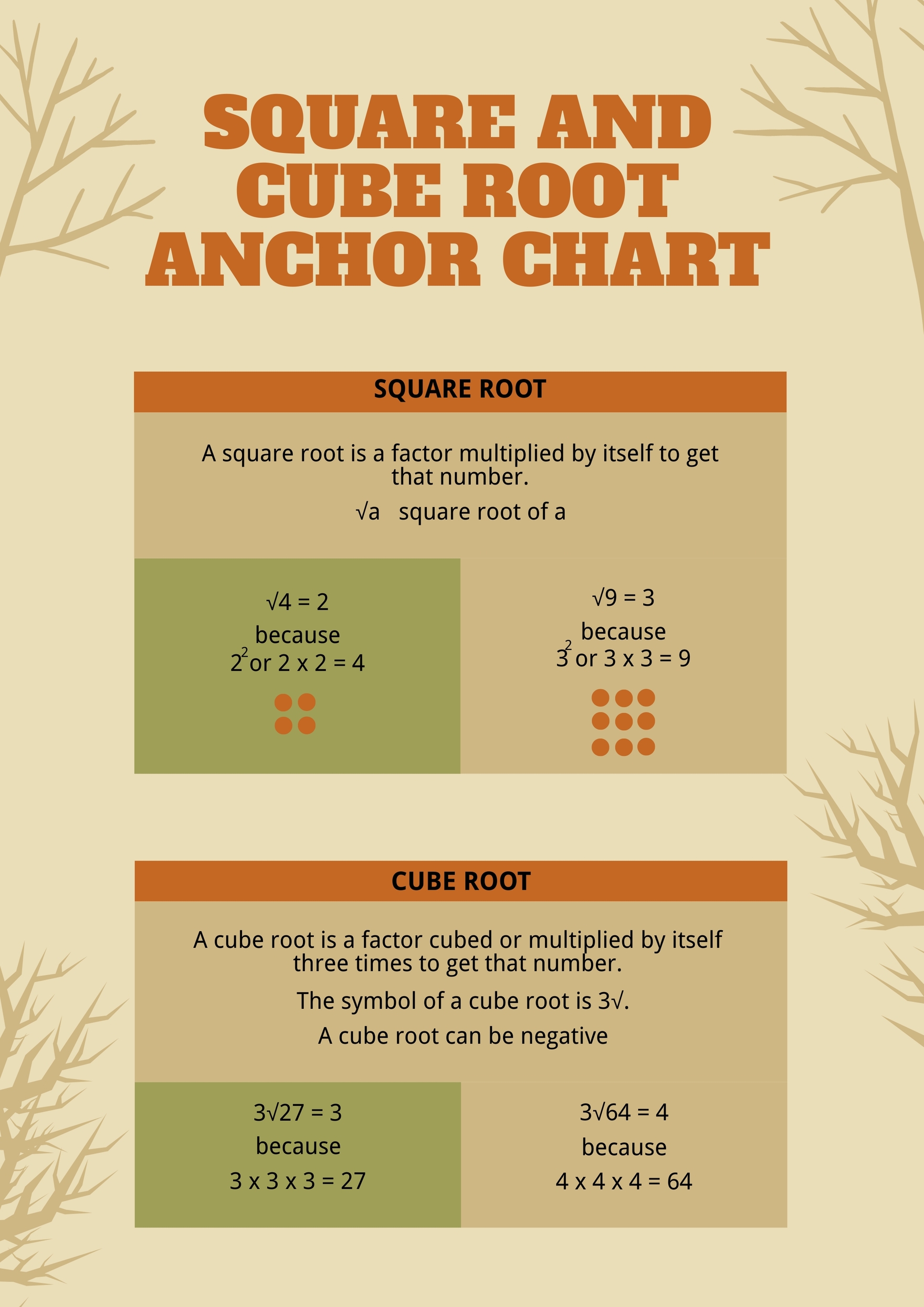
The Root Mean Square Velocity (Rms Velocity) Is A Way To Find A Single Velocity Value For The Particles.
The average velocity of gas particles is found using the root mean square velocity formula: How do i calculate root mean square velocity? Vᵣₘₛ = √(3 * r * t / m), where r is the gas constant, t is the temperature, and m is the molar mass. Woosh, your result is instantly computed!
Μ Rms = (3Rt/M) ½
Use the root mean square velocity calculator to find the velocity of a particle in a gas based on the kinetic theory of gases. To calculate, we need to: This online chemistry calculator may be used to calculate root mean square velocity, mean velocity (a.k.a average velocity) and median velocity. Our root mean square speed calculator gives you an effortless way to calculate the rms speed for an ideal and mostly monoatomic gases.