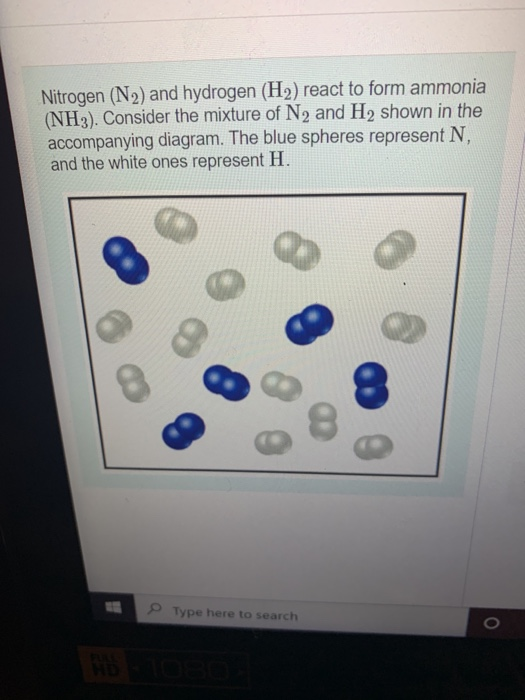
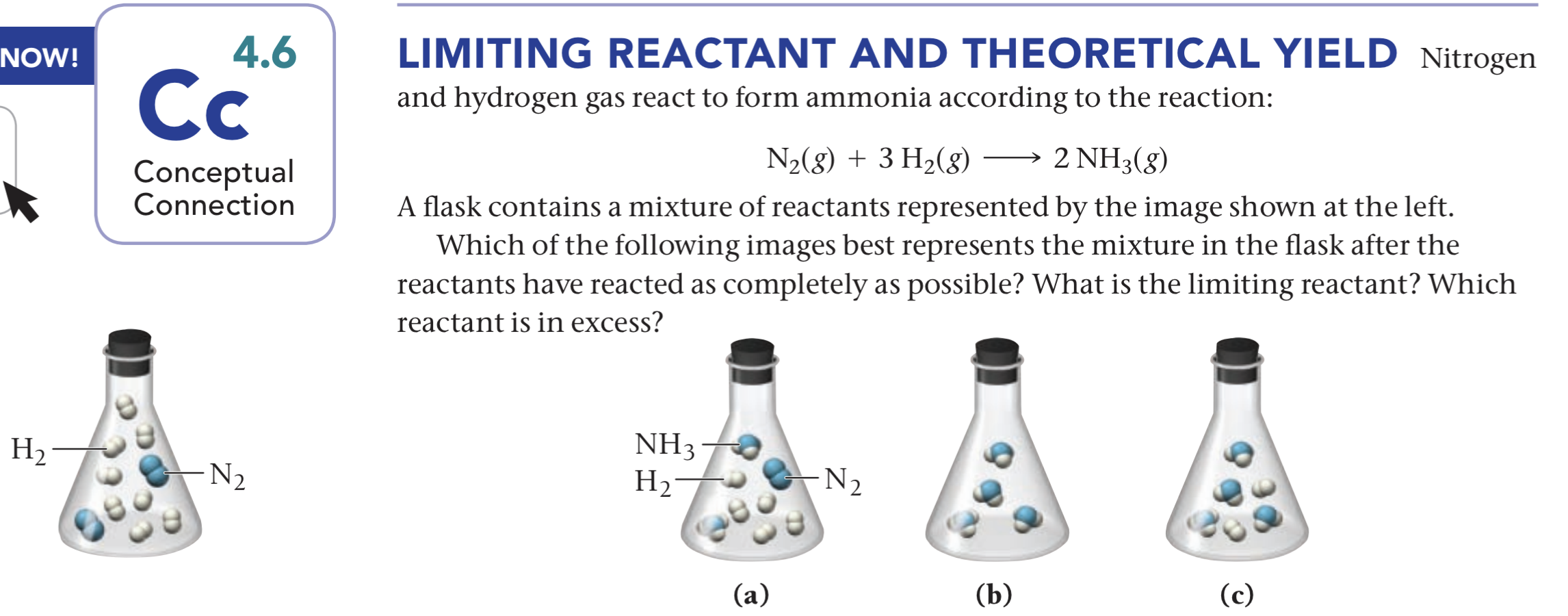
Nitrogen Gas And Hydrogen Gas React To Form Ammonia - This reaction is a fundamental chemical. The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. The balanced equation is shown. Nitrogen and hydrogen gases react to form ammonia gas as follows: When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃).
Nitrogen and hydrogen gases react to form ammonia gas as follows: The balanced equation is shown. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. This reaction is a fundamental chemical. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia.
This reaction is a fundamental chemical. Nitrogen and hydrogen gases react to form ammonia gas as follows: The balanced equation is shown. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃).
Nitrogen and hydrogen gases react to form ammonia gas as follows N2 ( g..
The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. This reaction is a fundamental chemical. The balanced equation is shown. Nitrogen and hydrogen gases react to form ammonia gas as follows: When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃).
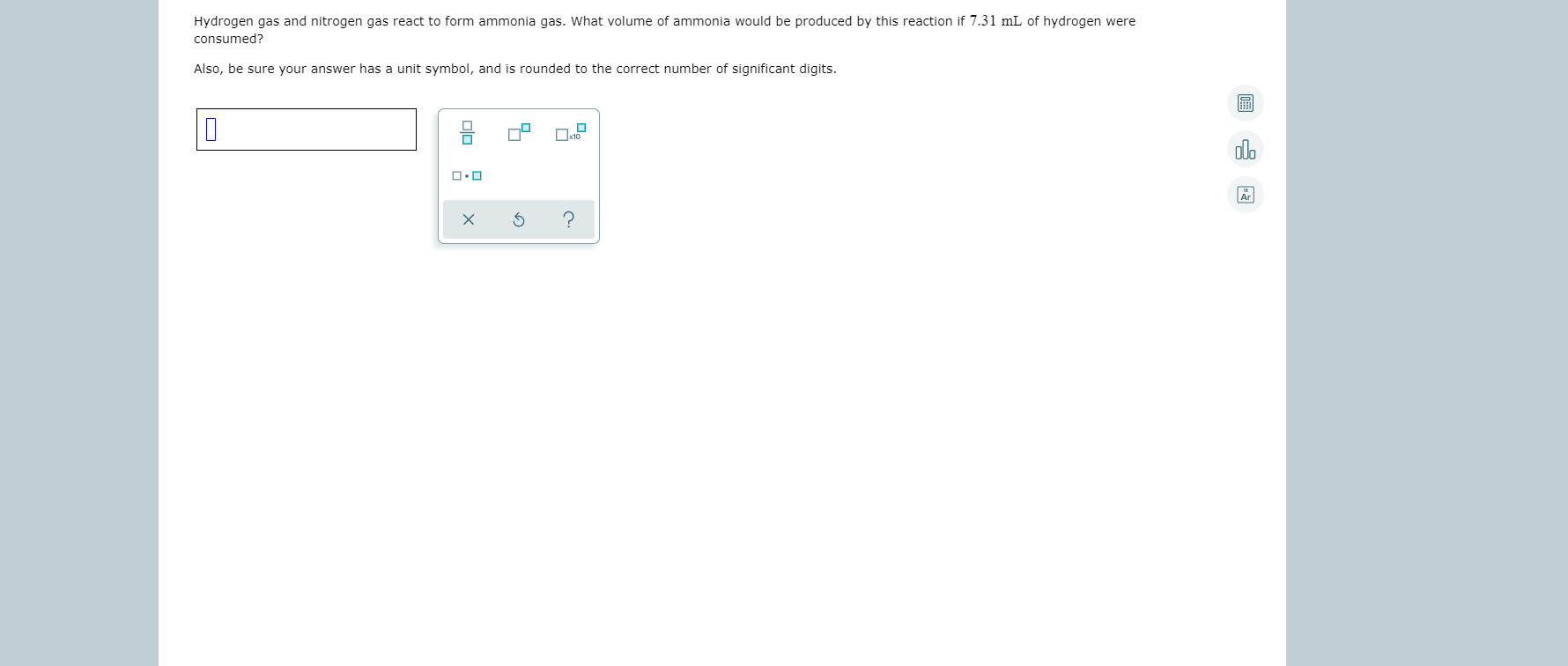
SOLVED Nitrogen gas and hydrogen gas react to form gaseous ammonia. If
$$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. Nitrogen and hydrogen gases react to form ammonia gas as follows: When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). The balanced equation is shown. The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas
The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. Nitrogen and hydrogen gases react to form ammonia gas as follows: This reaction is a fundamental chemical. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas
The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. The balanced equation is shown. This reaction is a fundamental chemical. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃).
Nitrogen Gas Reacts With Hydrogen Gas To Form Ammonia Printable Form
When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). Nitrogen and hydrogen gases react to form ammonia gas as follows: $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. This reaction is a fundamental chemical. The balanced equation is shown.
Hydrogen Gas Combines With Nitrogen To Form Ammonia
The balanced equation is shown. The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). Nitrogen and hydrogen gases react to form ammonia gas as follows: This reaction is a fundamental chemical.
SOLVED Hydrogen gas and nitrogen gas react to form ammonia gas What
The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. This reaction is a fundamental chemical. The balanced equation is shown. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. Nitrogen and hydrogen gases react to form ammonia gas as follows:
Answered Hydrogen gas and nitrogen gas react to… bartleby
Nitrogen and hydrogen gases react to form ammonia gas as follows: This reaction is a fundamental chemical. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃).
SOLVEDNitrogen and hydrogen gases react to form ammonia gas as follows
The balanced equation is shown. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. Nitrogen and hydrogen gases react to form ammonia gas as follows:
Nitrogen Gas And Hydrogen Gas React To Form Ammonia Gas Form example
$$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2. This reaction is a fundamental chemical. When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). The balanced equation is shown. The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia.
$$ \Mathrm{N}_{2}(G)+3 \Mathrm{H}_{2}(G) \Longrightarrow 2.
When nitrogen gas (n₂) reacts with hydrogen gas (h₂), they form ammonia gas (nh₃). The haber process is a reaction in which nitrogen gas is combined with hydrogen gas to form ammonia. This reaction is a fundamental chemical. The balanced equation is shown.