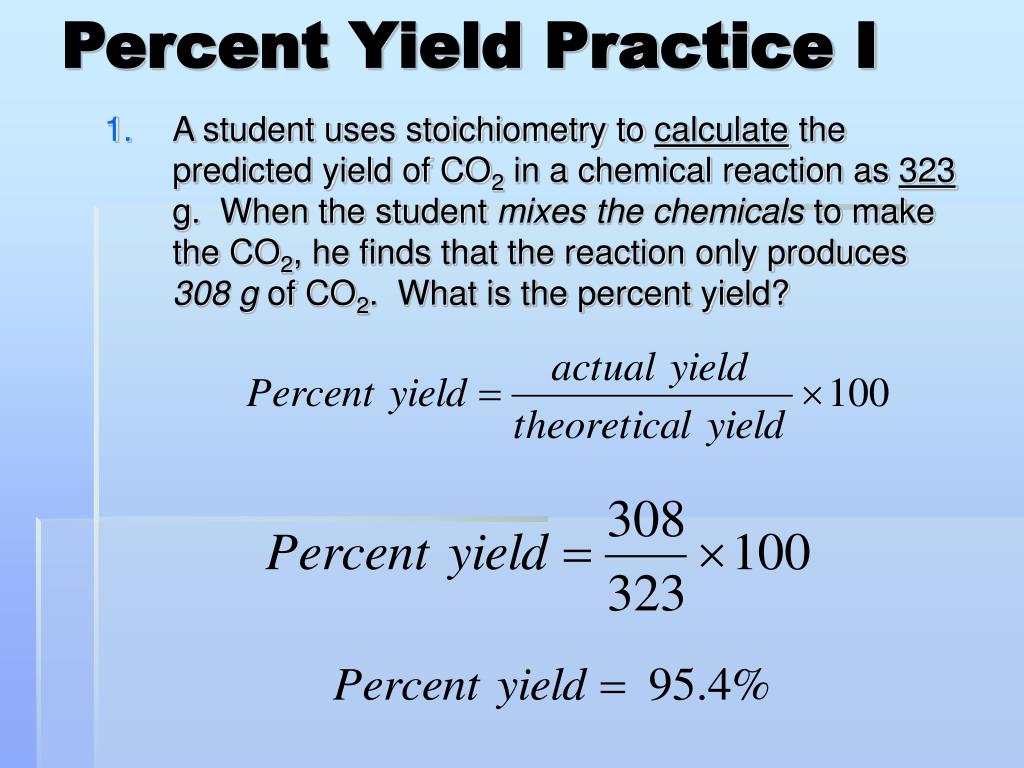
Percent Yield Worksheet Answers - 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Actual yield = given in the problem or the experimental yield. Assume the following hypothetical reaction takes place. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. 1) balance the equation for the reaction of iron (iii) phosphate with sodium. Percent yield calculation answers 1) balance this equation and state which of the six types of reaction is taking place: What is the percent yield for. 1 mg + 2 hno 3 1. Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide are formed, what is the percent yield?
Assume the following hypothetical reaction takes place. 1 mg + 2 hno 3 1. Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. 1) balance the equation for the reaction of iron (iii) phosphate with sodium. What is the percent yield for. Percent yield calculation answers 1) balance this equation and state which of the six types of reaction is taking place: 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide are formed, what is the percent yield? Actual yield = given in the problem or the experimental yield.
What is the percent yield for. 1 mg + 2 hno 3 1. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. Percent yield calculation answers 1) balance this equation and state which of the six types of reaction is taking place: Assume the following hypothetical reaction takes place. If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide are formed, what is the percent yield? 2a + 7b → 4c + 3d calculate the percentage yield in each of the. 1) balance the equation for the reaction of iron (iii) phosphate with sodium. Actual yield = given in the problem or the experimental yield.
Percent Yield Worksheet Sulfate Chemical Reactions
1) balance the equation for the reaction of iron (iii) phosphate with sodium. 1 mg + 2 hno 3 1. What is the percent yield for. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Percent yield calculation answers 1) balance this equation and state which.
Percent Yield Problems Worksheets
What is the percent yield for. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. Actual yield = given in the problem or the experimental yield. If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide are formed, what is the percent yield? Percent yield calculations practice problems 1) a reaction.
Free Printable Limiting Reactant and Percent Yield Worksheets
Actual yield = given in the problem or the experimental yield. Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. What is the percent yield for. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. Percent yield calculation answers 1) balance this equation and.
Quiz & Worksheet How to Calculate Percent Yield
Assume the following hypothetical reaction takes place. Actual yield = given in the problem or the experimental yield. What is the percent yield for. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. 1) balance the equation for the reaction of iron (iii) phosphate with sodium.
Worksheet Percent Yield
If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide are formed, what is the percent yield? 2a + 7b → 4c + 3d calculate the percentage yield in each of the. Actual yield = given in the problem or the experimental yield. 1 mg + 2 hno 3 1. 1) write a balanced equation for the.
Percent Yield Worksheet Answers Chemistry A Study Of Matter
1 mg + 2 hno 3 1. What is the percent yield for. Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. Actual yield = given in the problem or the experimental yield. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make.
Answers Percent Yield Practice PDF PDF Worksheets Library
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. 1 mg + 2 hno 3 1. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. Percent yield calculation answers 1) balance this equation and state which of the six types.
Percent Yield Worksheet With Answers
Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide are formed, what is the percent yield? 2a + 7b → 4c + 3d calculate the percentage yield in each of the. Percent yield calculation answers 1) balance.
Quiz & Worksheet Calculating Reaction Yield and Percentage Yield from
2a + 7b → 4c + 3d calculate the percentage yield in each of the. Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. What is the percent yield for. 1 mg + 2 hno 3 1. If 35.0 grams of bromine are reacted and 27.9 grams of phosphorous tribromide.
Percent Yield Practice Problems Chemistry
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Assume the following hypothetical reaction takes place. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. 1 mg + 2 hno 3 1. 1) balance the equation for the reaction of.
If 35.0 Grams Of Bromine Are Reacted And 27.9 Grams Of Phosphorous Tribromide Are Formed, What Is The Percent Yield?
1 mg + 2 hno 3 1. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. 2a + 7b → 4c + 3d calculate the percentage yield in each of the. 1) balance the equation for the reaction of iron (iii) phosphate with sodium.
Percent Yield Calculation Answers 1) Balance This Equation And State Which Of The Six Types Of Reaction Is Taking Place:
Percent yield calculations practice problems 1) a reaction with a·calculated yield of 9.23 g produced 7.89 g of product. Actual yield = given in the problem or the experimental yield. Assume the following hypothetical reaction takes place. What is the percent yield for.