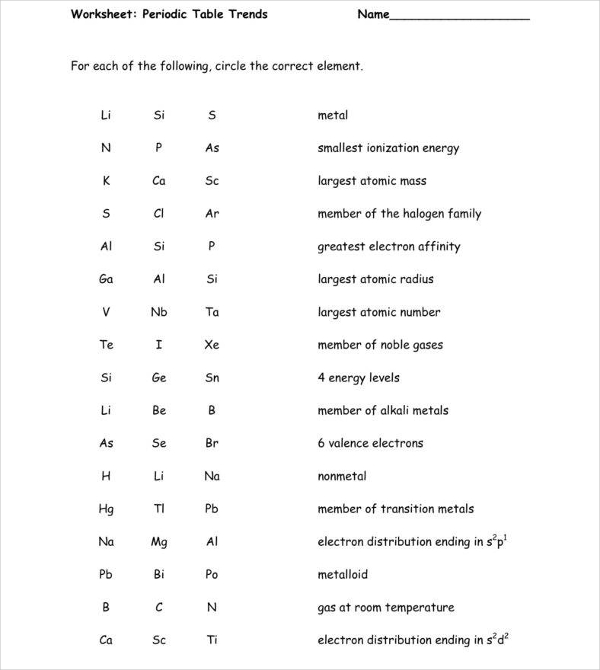
Periodic Trends Worksheet Pdf - Give two examples of elements for each category. Periodic trends atomic radius 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? What trends do you notice for the atomic radii of period 3? The atomic radius gets smaller as atomic number increases. Discuss the importance of mendeleev’s periodic law. Explain why this trend occurs. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Identify each element as a metal, metalloid, or nonmetal. Going from left to right across a.
What trends do you notice for the atomic radii of period 3? Periodic trends atomic radius 1. Going from left to right across a. Give two examples of elements for each category. Explain why this trend occurs. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. What trend in atomic radius do you see as you go down a group/family on the periodic table? The atomic radius gets smaller as atomic number increases. Discuss the importance of mendeleev’s periodic law. Identify each element as a metal, metalloid, or nonmetal.
Periodic trends atomic radius 1. Explain why this trend occurs. What trend in atomic radius do you see as you go down a group/family on the periodic table? The atomic radius gets smaller as atomic number increases. Going from left to right across a. Identify each element as a metal, metalloid, or nonmetal. Discuss the importance of mendeleev’s periodic law. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. What trends do you notice for the atomic radii of period 3? Give two examples of elements for each category.
Worksheet Periodic Table Trends Englishworksheet.my.id
Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Going from left to right across a. The atomic radius gets smaller as atomic number increases. What trends do you notice for the atomic radii of period 3? Give two examples of elements for each category.
Periodic Table Review Worksheet Pdf Matttroy
Going from left to right across a. Periodic trends atomic radius 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? The atomic radius gets smaller as atomic number increases. Discuss the importance of mendeleev’s periodic law.
Periodic Trends Worksheet PDF Chemical Elements Ion
Give two examples of elements for each category. Explain why this trend occurs. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Discuss the importance of mendeleev’s periodic law. Identify each element as a metal, metalloid, or nonmetal.
PeriodicTrendsWorksheet PDF
The atomic radius gets smaller as atomic number increases. What trend in atomic radius do you see as you go down a group/family on the periodic table? What trends do you notice for the atomic radii of period 3? Periodic trends atomic radius 1. Identify each element as a metal, metalloid, or nonmetal.
Free worksheet periodic table trends answer key, Download Free
Identify each element as a metal, metalloid, or nonmetal. Going from left to right across a. What trends do you notice for the atomic radii of period 3? The atomic radius gets smaller as atomic number increases. Discuss the importance of mendeleev’s periodic law.
Periodic Trends Worksheet printable pdf download
Give two examples of elements for each category. Periodic trends atomic radius 1. Discuss the importance of mendeleev’s periodic law. Explain why this trend occurs. The atomic radius gets smaller as atomic number increases.
Worksheets Periodic Table Trends
Give two examples of elements for each category. Periodic trends atomic radius 1. Explain why this trend occurs. Identify each element as a metal, metalloid, or nonmetal. Going from left to right across a.
Worksheet Periodic Table Trends Zip Worksheet
Discuss the importance of mendeleev’s periodic law. Going from left to right across a. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Periodic trends atomic radius 1. Identify each element as a metal, metalloid, or nonmetal.
Worksheet Periodic Trends Answers
Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Identify each element as a metal, metalloid, or nonmetal. The atomic radius gets smaller as atomic number increases. What trends do you notice for the atomic radii of period 3? Give two examples of elements for each category.
Free Printable Periodic Trends Worksheets
Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. The atomic radius gets smaller as atomic number increases. Explain why this trend occurs. Periodic trends atomic radius 1. What trends do you notice for the atomic radii of period 3?
What Trend In Atomic Radius Do You See As You Go Down A Group/Family On The Periodic Table?
Going from left to right across a. Discuss the importance of mendeleev’s periodic law. Give two examples of elements for each category. Explain why this trend occurs.
What Trends Do You Notice For The Atomic Radii Of Period 3?
Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. The atomic radius gets smaller as atomic number increases. Periodic trends atomic radius 1. Identify each element as a metal, metalloid, or nonmetal.