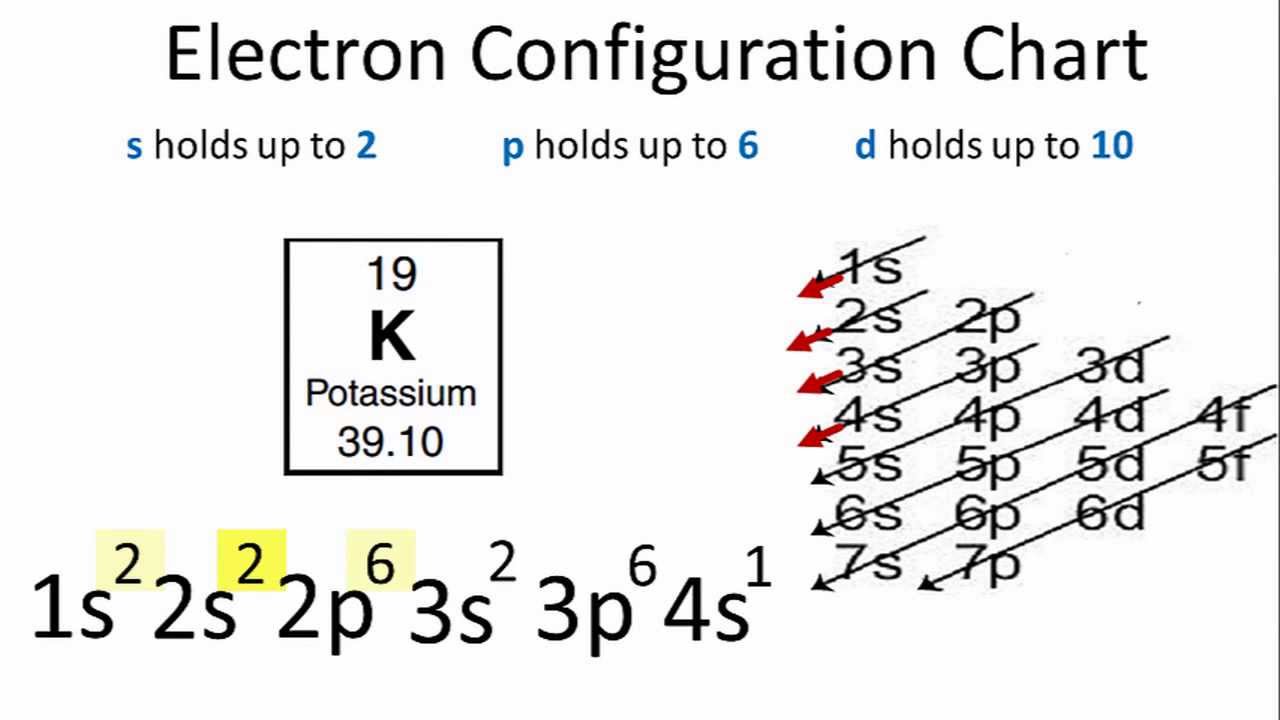
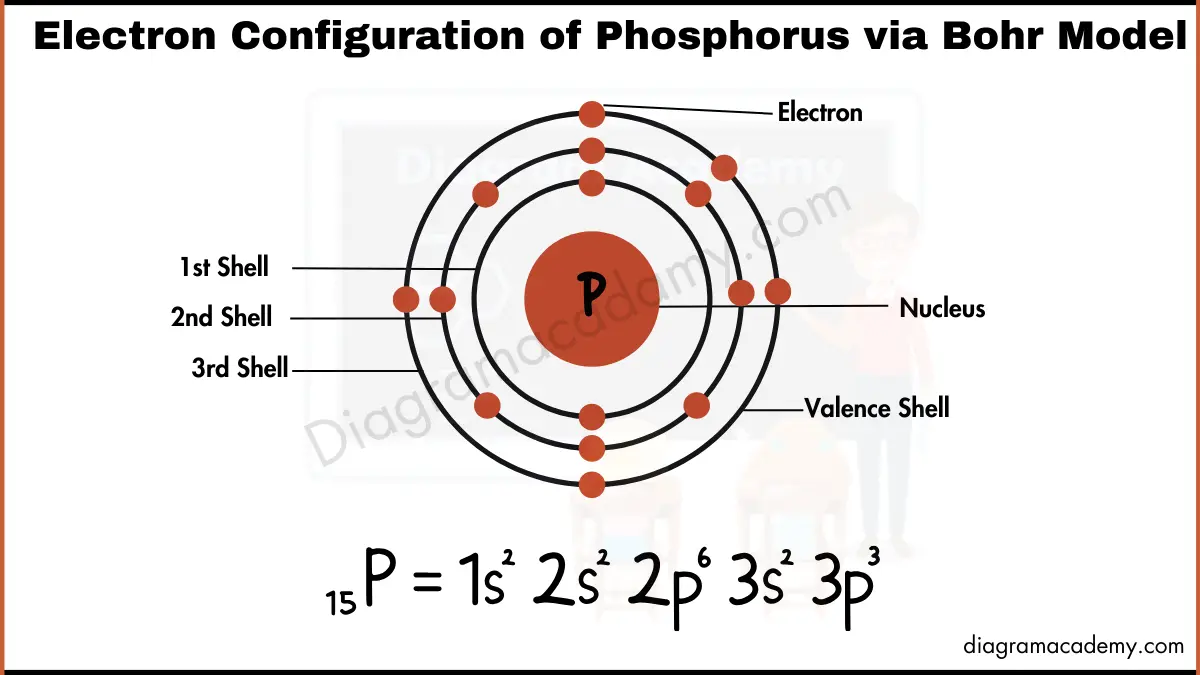
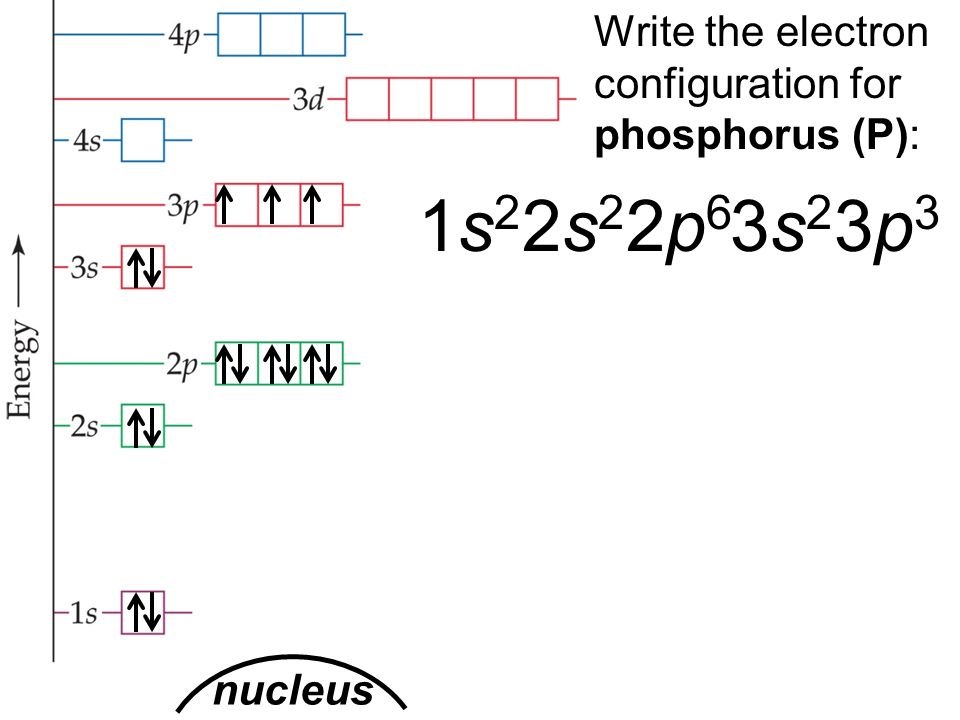
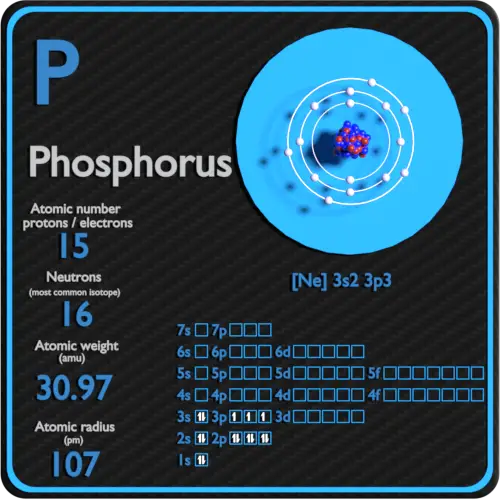
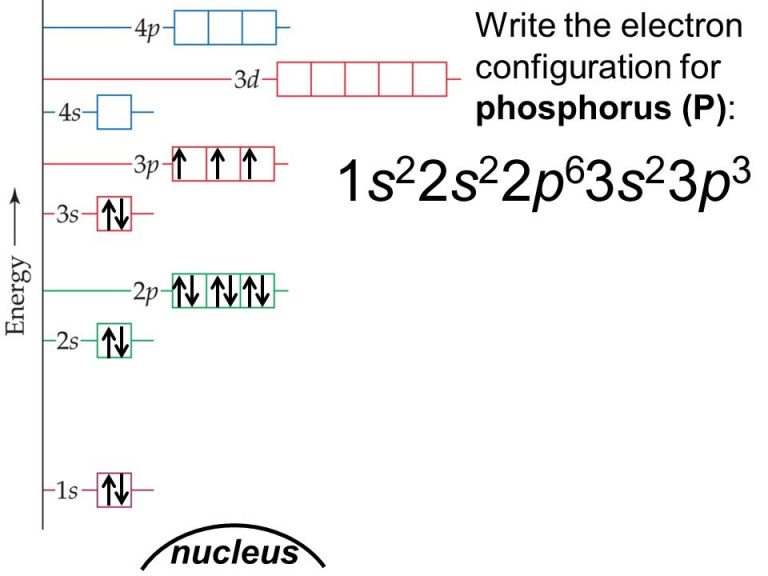
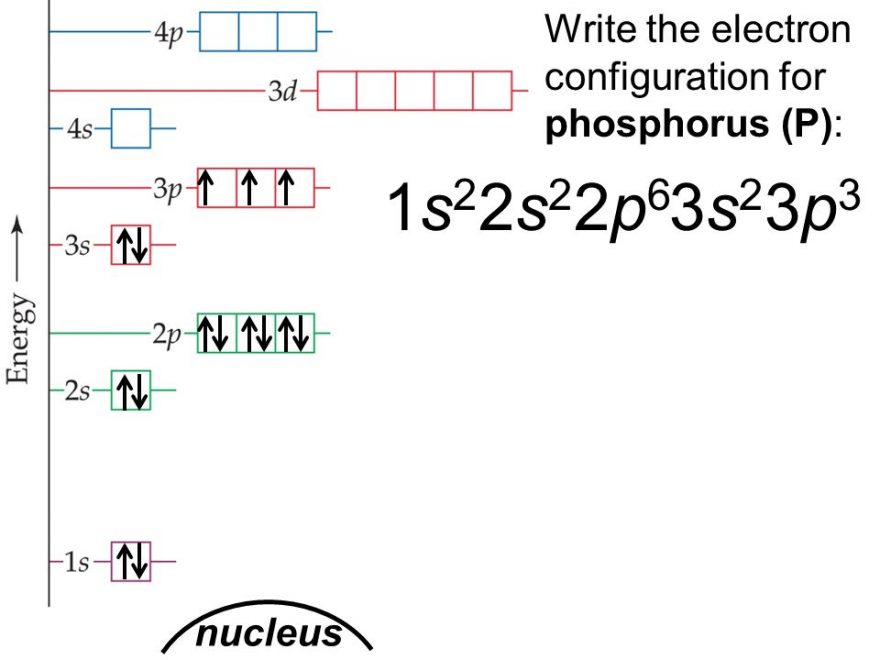
Phosphorus Electron Configuration Long Form - Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15. Electronic configuration of the phosphorus atom. Be sure to label the subshells in order of energy, with.
In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. Electronic configuration of the phosphorus atom. Be sure to label the subshells in order of energy, with.
Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. Electronic configuration of the phosphorus atom. Be sure to label the subshells in order of energy, with. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15.
😍 Electron configuration examples. Abbreviated Electron configurations
Be sure to label the subshells in order of energy, with. Electronic configuration of the phosphorus atom. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons.
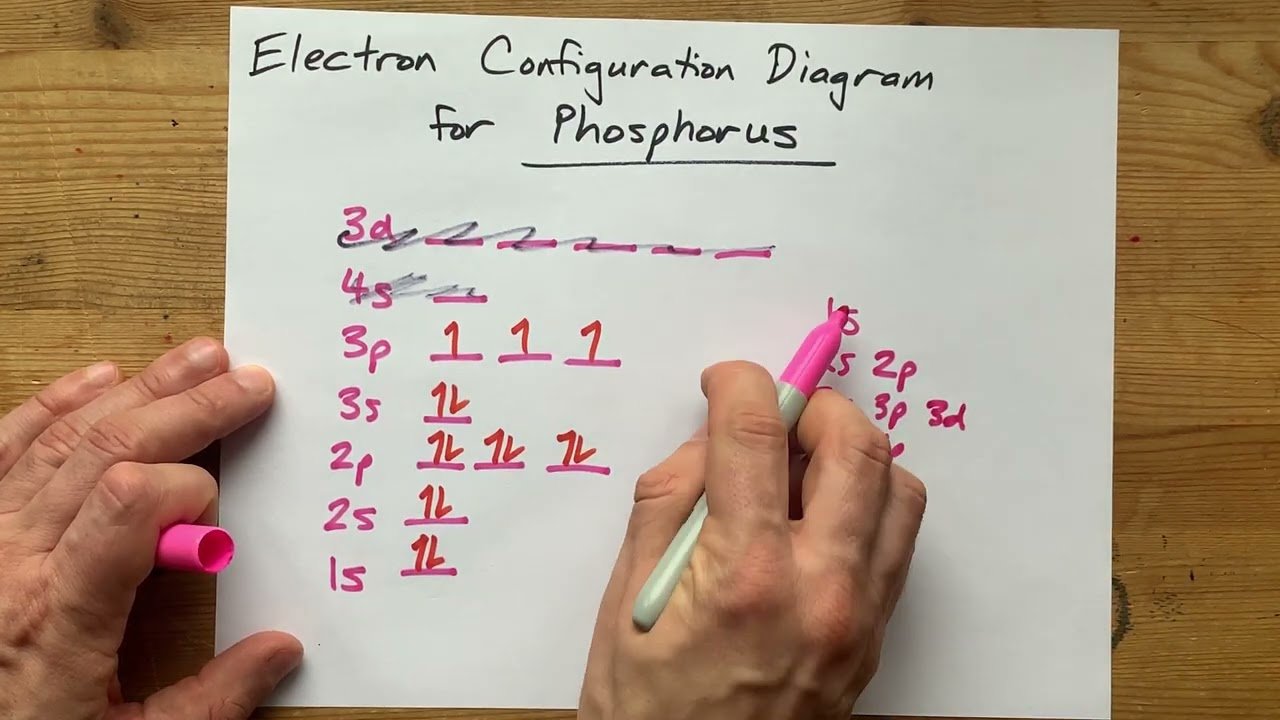
Electronic Configuration of Phosphorus Diagram
Electronic configuration of the phosphorus atom. Be sure to label the subshells in order of energy, with. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an.
nucleus. Dynamic Periodic Table of Elements and Chemistry
Be sure to label the subshells in order of energy, with. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are.
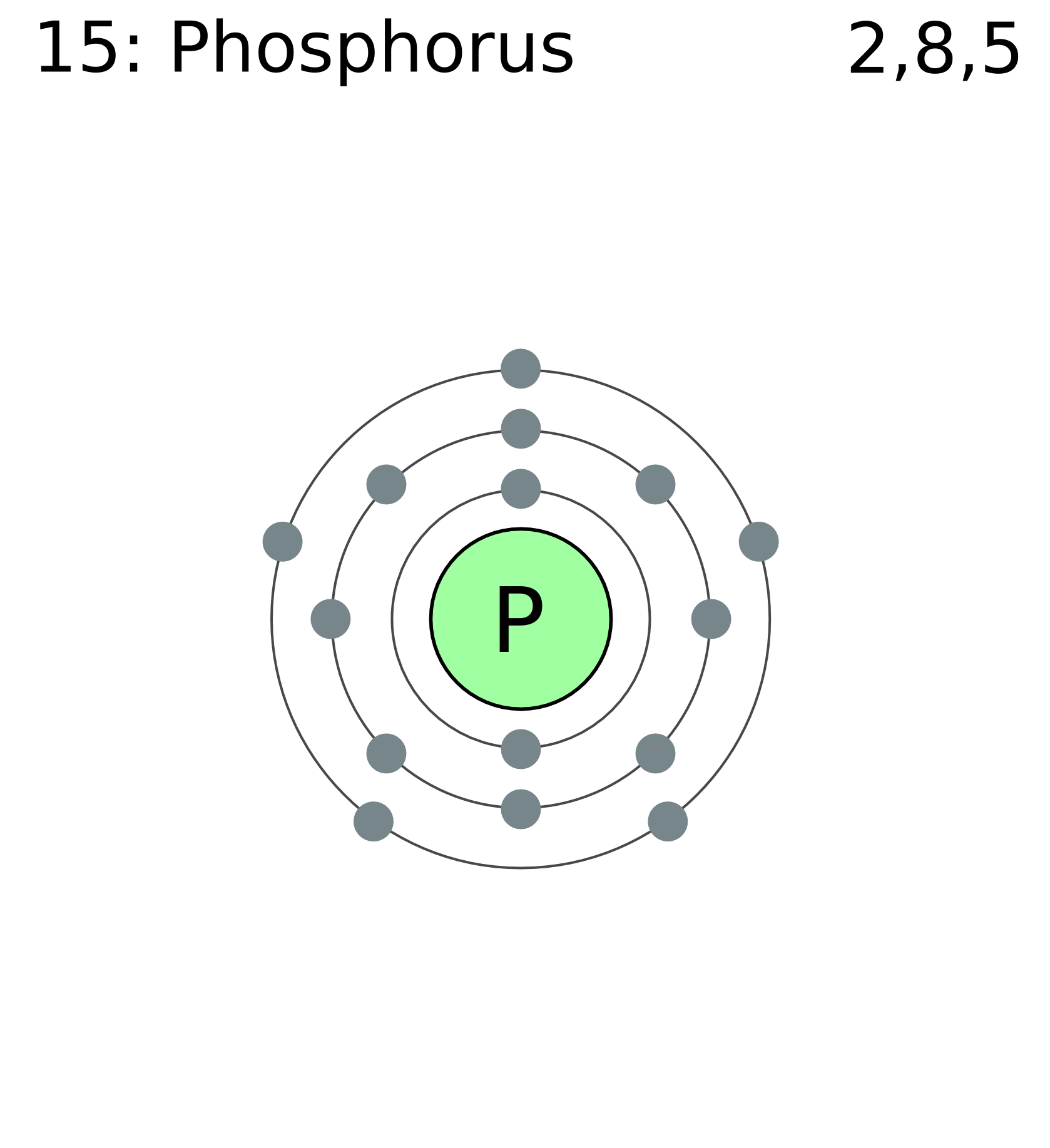
Phosphorus Protons Neutrons Electrons Electron Configuration
Electronic configuration of the phosphorus atom. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. Be sure to label the subshells in order of energy, with. In order to write the phosphorus electron configuration we first need to know the number of electrons.
Phosphorus Electron Configuration (P) with Orbital Diagram
In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15. Electronic configuration of the phosphorus atom. Be sure to label the subshells in order of energy, with. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an.
Phosphorus Electron Configuration (P) with Orbital Diagram
Be sure to label the subshells in order of energy, with. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are.
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
Electronic configuration of the phosphorus atom. Be sure to label the subshells in order of energy, with. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons.
Phosphorus Orbital diagram, Electron configuration, and Valence electrons
Be sure to label the subshells in order of energy, with. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are.
Phosphorus Electron Configuration (P) with Orbital Diagram
Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. Be sure to label the subshells in order of energy, with. Electronic configuration of the phosphorus atom. In order to write the phosphorus electron configuration we first need to know the number of electrons.
The Electron Configuration of Phosphorus Unveiling its Atomic
Be sure to label the subshells in order of energy, with. Electronic configuration of the phosphorus atom. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15. Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an.
Electronic Configuration Of The Phosphorus Atom.
Phosphorus is a chemical element of the periodic table with chemical symbol p and atomic number 15 with an atomic weight of 30.9738 u and is. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15. Be sure to label the subshells in order of energy, with.