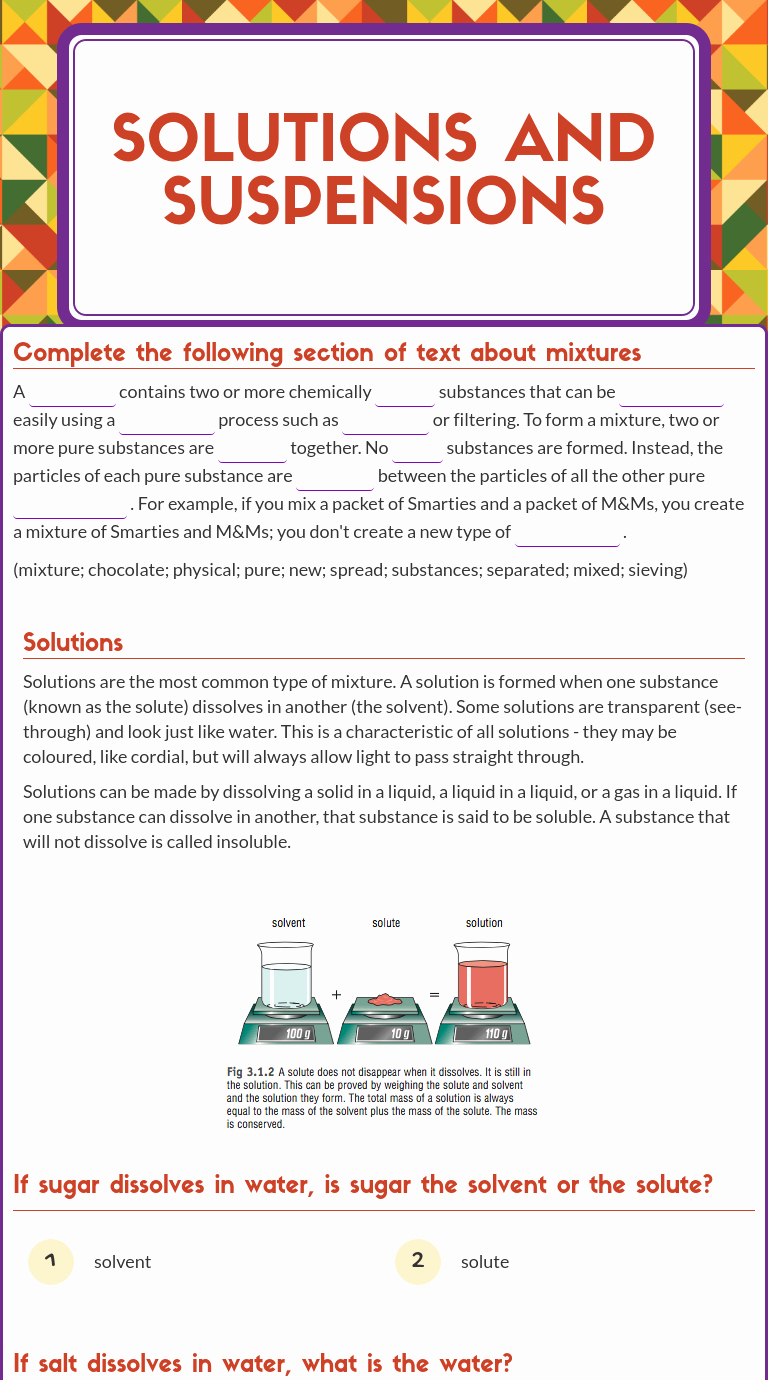
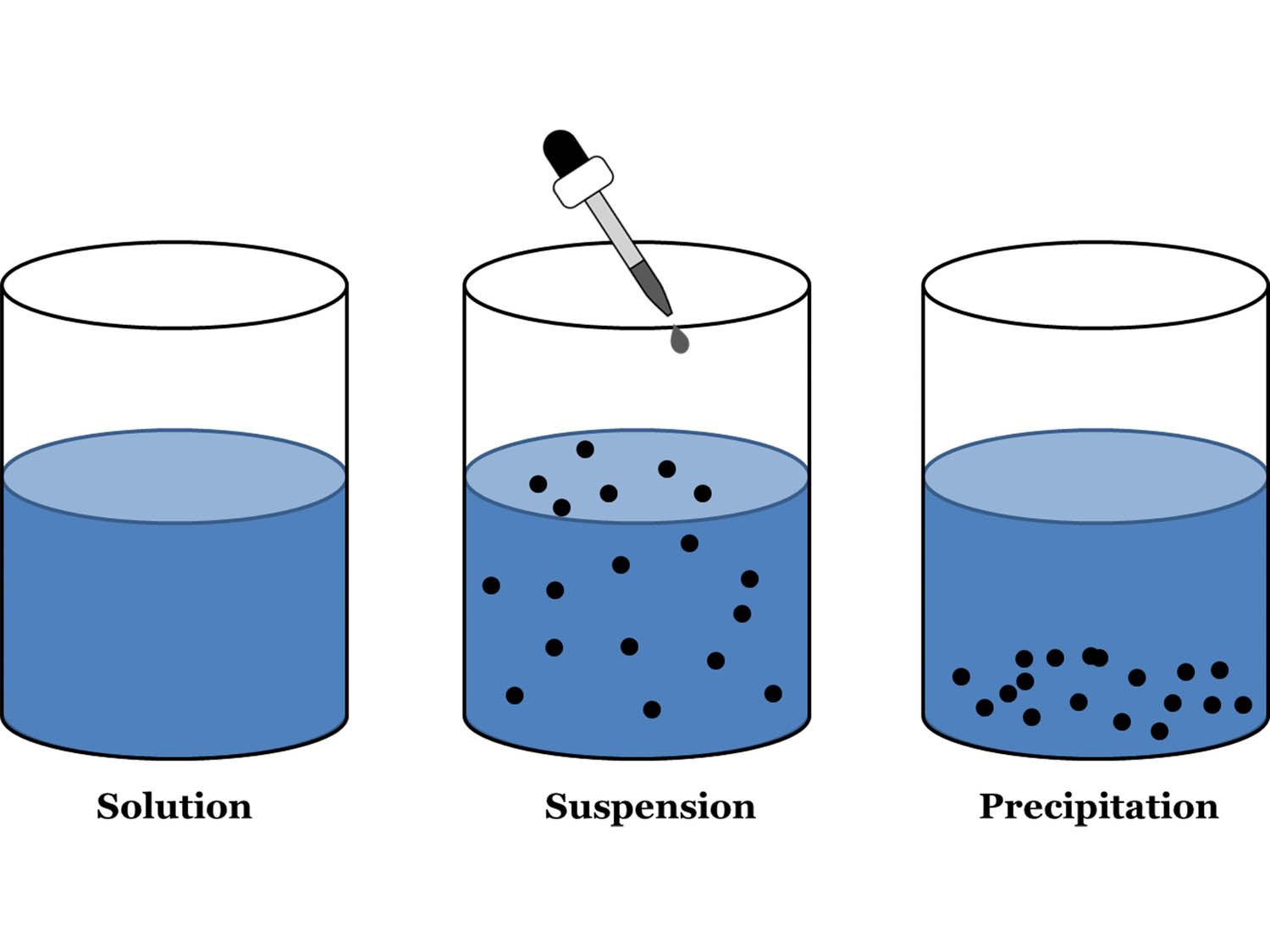
Solutions Colloids And Suspensions Worksheet - Given an unknown mixture consisting of two substances, explain how a scientist could use. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a colloid, one substance forms clumps of particles in the other substance. Which of the following decreases the average kinetic energy of solvent molecules?. In a solution, the particles of two substances are mixed together completely evenly, solutions.
In a colloid, one substance forms clumps of particles in the other substance. In a solution, the particles of two substances are mixed together completely evenly, solutions. Given an unknown mixture consisting of two substances, explain how a scientist could use. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. Which of the following decreases the average kinetic energy of solvent molecules?.
Given an unknown mixture consisting of two substances, explain how a scientist could use. Which of the following decreases the average kinetic energy of solvent molecules?. In a colloid, one substance forms clumps of particles in the other substance. In a solution, the particles of two substances are mixed together completely evenly, solutions. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:.
50 Solutions Colloids And Suspensions Worksheet
In a colloid, one substance forms clumps of particles in the other substance. Which of the following decreases the average kinetic energy of solvent molecules?. Given an unknown mixture consisting of two substances, explain how a scientist could use. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a solution, the particles of two substances.
Solutions Suspensions And Colloids Quiz
Which of the following decreases the average kinetic energy of solvent molecules?. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a colloid, one substance forms clumps of particles in the other substance. In a solution, the particles of two substances are mixed together completely evenly, solutions. Given an unknown mixture consisting of two substances,.
Solutions Colloids And Suspensions Worksheet
Given an unknown mixture consisting of two substances, explain how a scientist could use. In a solution, the particles of two substances are mixed together completely evenly, solutions. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. Which of the following decreases the average kinetic energy of solvent molecules?. In a colloid, one substance forms clumps.
Solutions Colloids and Suspensions Worksheet Fresh solutions Colloids
In a solution, the particles of two substances are mixed together completely evenly, solutions. Which of the following decreases the average kinetic energy of solvent molecules?. Given an unknown mixture consisting of two substances, explain how a scientist could use. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a colloid, one substance forms clumps.
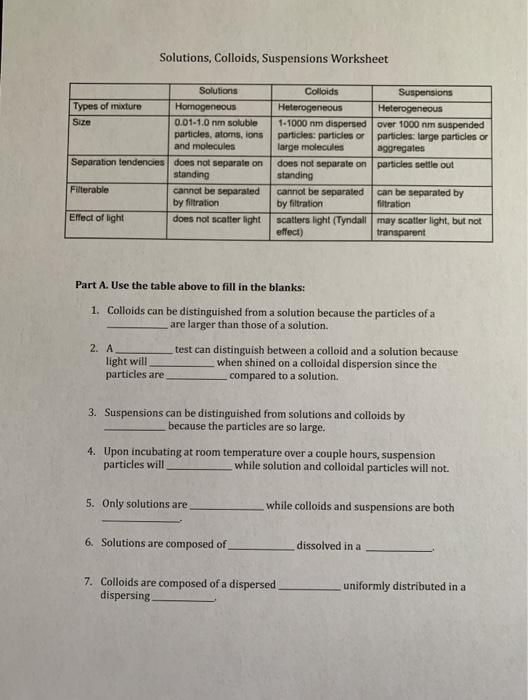
Solved Solutions, Colloids, and Suspensions Worksheet Table
In a colloid, one substance forms clumps of particles in the other substance. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a solution, the particles of two substances are mixed together completely evenly, solutions. Which of the following decreases the average kinetic energy of solvent molecules?. Given an unknown mixture consisting of two substances,.
Solutions Colloids And Suspensions Worksheet E Street Light
Which of the following decreases the average kinetic energy of solvent molecules?. Given an unknown mixture consisting of two substances, explain how a scientist could use. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a colloid, one substance forms clumps of particles in the other substance. In a solution, the particles of two substances.
50 Solutions Colloids And Suspensions Worksheet
In a colloid, one substance forms clumps of particles in the other substance. In a solution, the particles of two substances are mixed together completely evenly, solutions. Which of the following decreases the average kinetic energy of solvent molecules?. Given an unknown mixture consisting of two substances, explain how a scientist could use. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee.
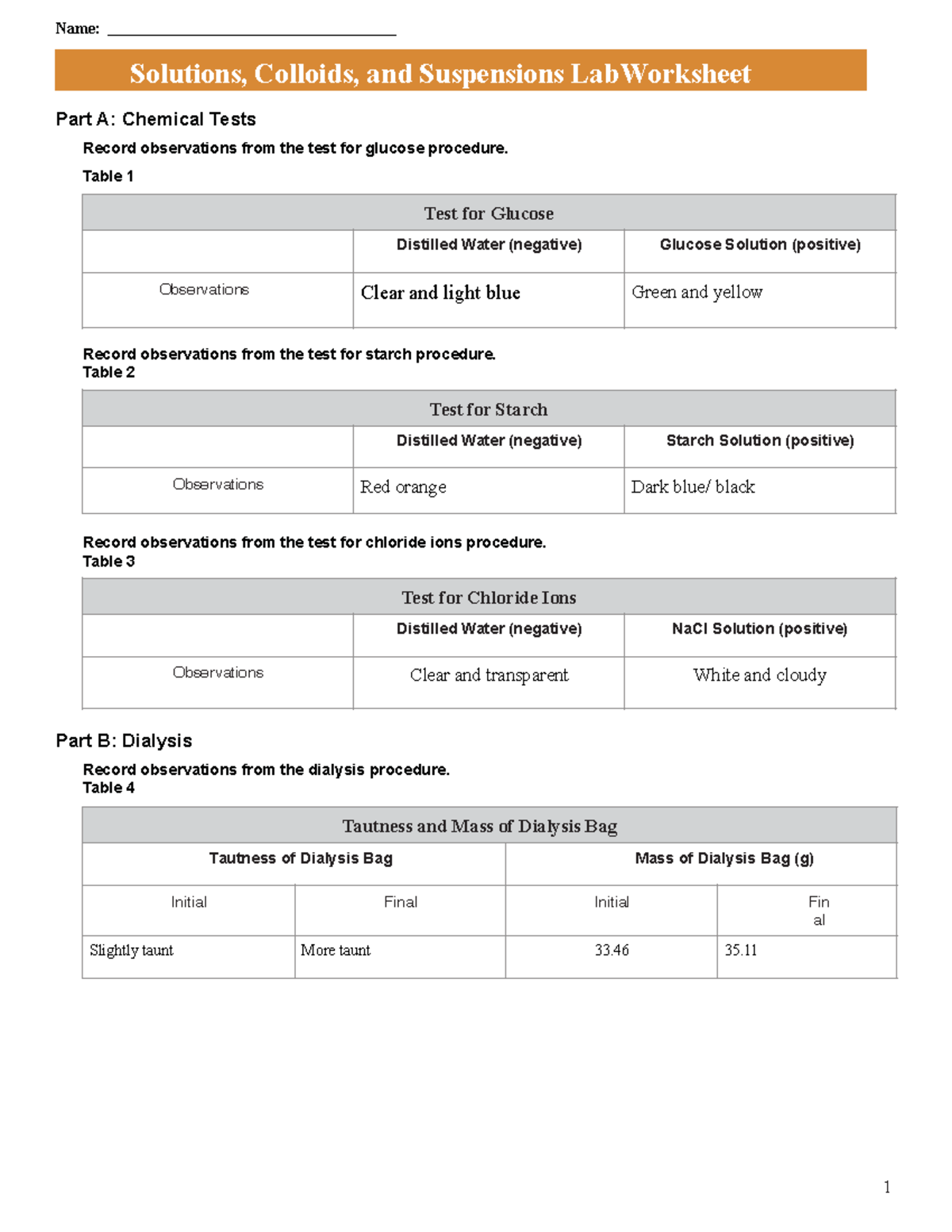
CHM 101L Solutions, Colloids, and Suspensions Lab Worksheet
Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a solution, the particles of two substances are mixed together completely evenly, solutions. Given an unknown mixture consisting of two substances, explain how a scientist could use. In a colloid, one substance forms clumps of particles in the other substance. Which of the following decreases the.
Solved Solutions, Colloids, Suspensions Worksheet Solutions
In a solution, the particles of two substances are mixed together completely evenly, solutions. Which of the following decreases the average kinetic energy of solvent molecules?. Given an unknown mixture consisting of two substances, explain how a scientist could use. In a colloid, one substance forms clumps of particles in the other substance. Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee.
Solutions Colloids And Suspensions Worksheet
Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a colloid, one substance forms clumps of particles in the other substance. Which of the following decreases the average kinetic energy of solvent molecules?. In a solution, the particles of two substances are mixed together completely evenly, solutions. Given an unknown mixture consisting of two substances,.
Which Of The Following Decreases The Average Kinetic Energy Of Solvent Molecules?.
Activity 3 solutions, suspensions, and colloids 7hat$o9ou3ee goals in this activity you will:. In a solution, the particles of two substances are mixed together completely evenly, solutions. In a colloid, one substance forms clumps of particles in the other substance. Given an unknown mixture consisting of two substances, explain how a scientist could use.