What Are Three Ways To Increase The Rate Of Solvation - There are several factors that effect the rate of solvation. There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. To increase the rate of solvation, you need to. Solvation occurs when solute and solvent particles come in contact with each other. What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which the solute. Temperature, concentration, surface area of solute,. To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing.
To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. There are several factors that effect the rate of solvation. To increase the rate of solvation, you need to. There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which the solute. Solvation occurs when solute and solvent particles come in contact with each other. Temperature, concentration, surface area of solute,.
Solvation occurs when solute and solvent particles come in contact with each other. There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. To increase the rate of solvation, you need to. There are several factors that effect the rate of solvation. What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which the solute. To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. Temperature, concentration, surface area of solute,.
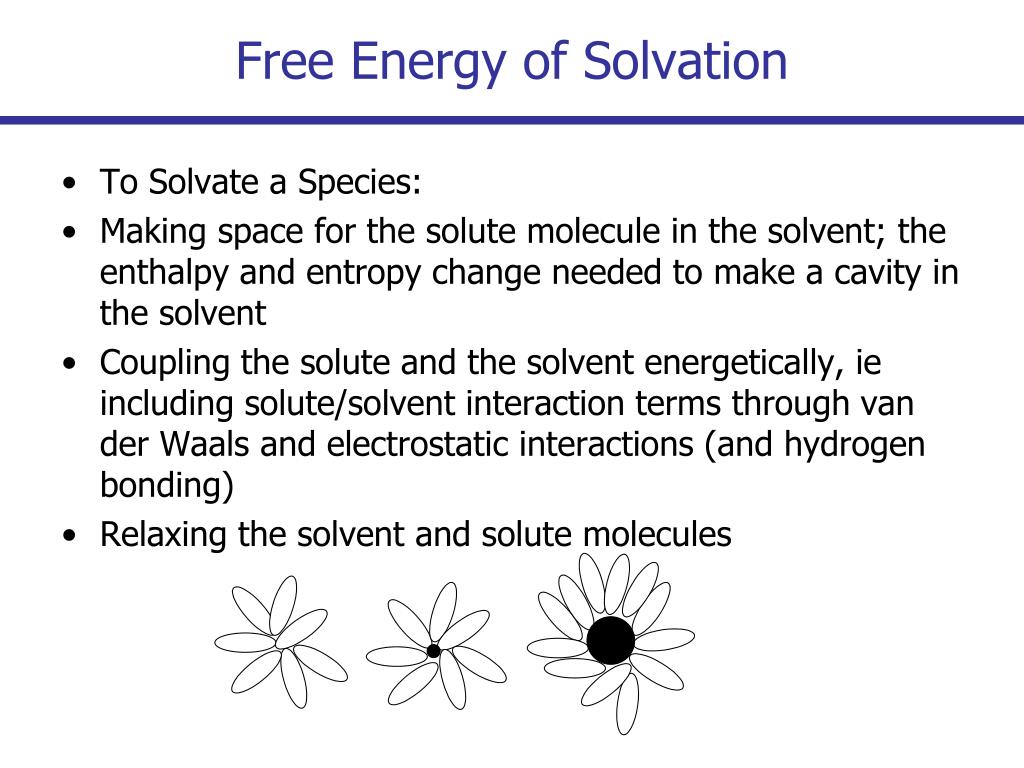
Approach to the solvation energy, which includes the solvent cavity
To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. There are several factors that effect the rate of solvation. Solvation occurs when solute and solvent particles come in contact with each other. To increase the rate of solvation, you need to. What are the three.
CompChem.06.02 Solvation Models Explicit Solvent solvation คือ
To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. Temperature, concentration, surface area of solute,. To increase the rate of solvation, you need to. Solvation occurs when solute and solvent particles come in contact with each other. There are three things that can be specifically.
why does the conductance decreases with the increase in solvation?
To increase the rate of solvation, you need to. Temperature, concentration, surface area of solute,. To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which.
An illustration of the three solvent regimes that are considered for
There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. There are several factors that effect the rate of solvation. What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which the solute. To increase the.
The performances of all combinations of three solvation levels and six
Solvation occurs when solute and solvent particles come in contact with each other. To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which the solute..
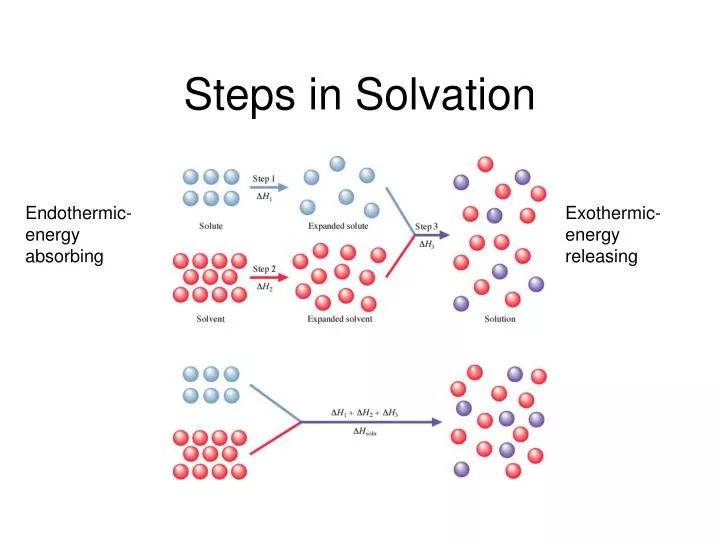
PPT Steps in Solvation PowerPoint Presentation, free download ID
Solvation occurs when solute and solvent particles come in contact with each other. To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. To increase the rate of solvation, you need to. There are three things that can be specifically done to increase the rate of.
(a) Schematical illustration of evolution of solvation structures with
There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. Solvation occurs when solute and solvent particles come in contact with each other. There are several factors that effect the rate of solvation. To increase the rate of solvation, one can (1) raise the temperature of.
Rates of Solvation lab
To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases the kinetic energy of the molecules, allowing. Solvation occurs when solute and solvent particles come in contact with each other. There are several factors that effect the rate of solvation. What are the three common ways to increase collisions between solute and solvent.
PPT Solvation PowerPoint Presentation, free download ID5189992
Temperature, concentration, surface area of solute,. There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. Solvation occurs when solute and solvent particles come in contact with each other. To increase the rate of solvation, one can (1) raise the temperature of the solvent, which increases.
Name One Way to Decrease the Rate of Solvation Sciencing
Temperature, concentration, surface area of solute,. There are several factors that effect the rate of solvation. There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent. Solvation occurs when solute and solvent particles come in contact with each other. What are the three common ways to.
Temperature, Concentration, Surface Area Of Solute,.
What are the three common ways to increase collisions between solute and solvent particles and thus increase the rate at which the solute. There are several factors that effect the rate of solvation. To increase the rate of solvation, you need to. There are three things that can be specifically done to increase the rate of solution of a solid and soluble solute in a solvent.
To Increase The Rate Of Solvation, One Can (1) Raise The Temperature Of The Solvent, Which Increases The Kinetic Energy Of The Molecules, Allowing.
Solvation occurs when solute and solvent particles come in contact with each other.