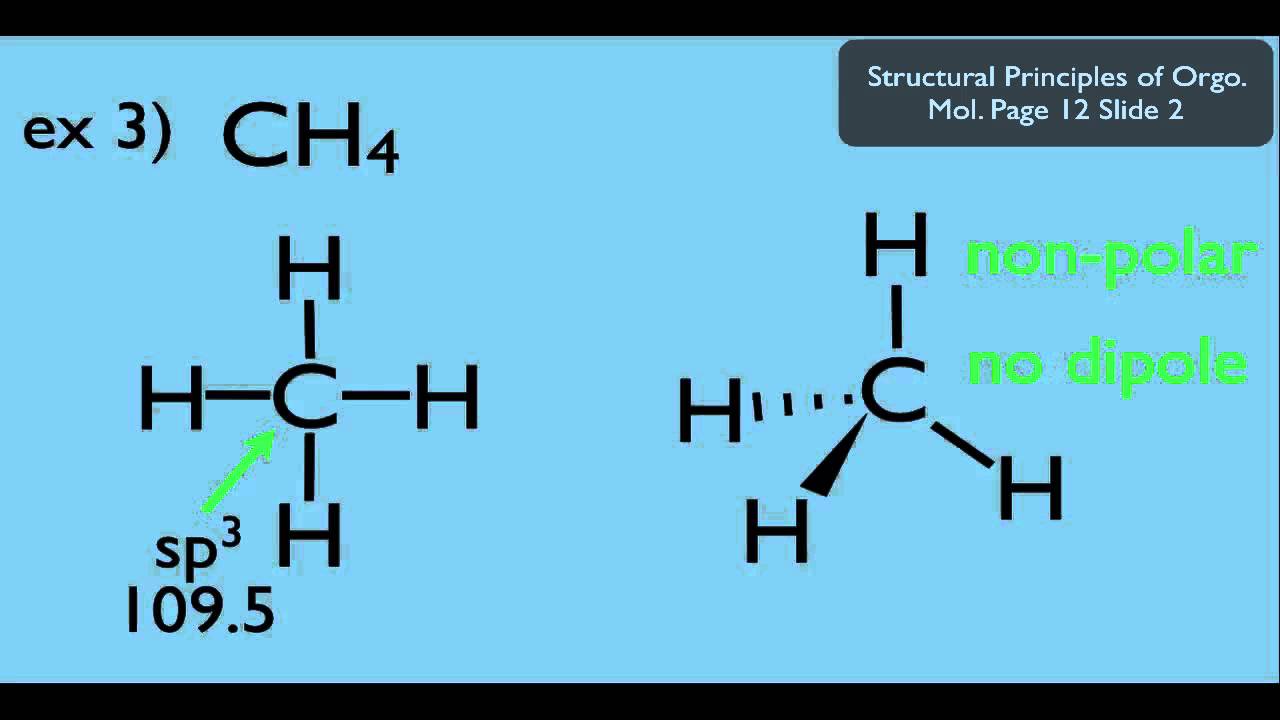
What Causes A Molecule To Have A Net Dipole Moment - Dipole moments occur when there is a separation of charge. A net dipole moment in a molecule exists when there is an uneven distribution of electron. A molecule possesses a net dipole moment when polar covalent bonds create an. Dipole moment (μ μ) is the measure of net molecular polarity, which is the.
A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge. Dipole moment (μ μ) is the measure of net molecular polarity, which is the.
A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge. Dipole moment (μ μ) is the measure of net molecular polarity, which is the.
Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps
Dipole moment (μ μ) is the measure of net molecular polarity, which is the. Dipole moments occur when there is a separation of charge. A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron.
Find The Molecule With A Net Dipole Moment
A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge. Dipole moment (μ μ) is the measure of net molecular polarity, which is the. A molecule possesses a net dipole moment when polar covalent bonds create an.
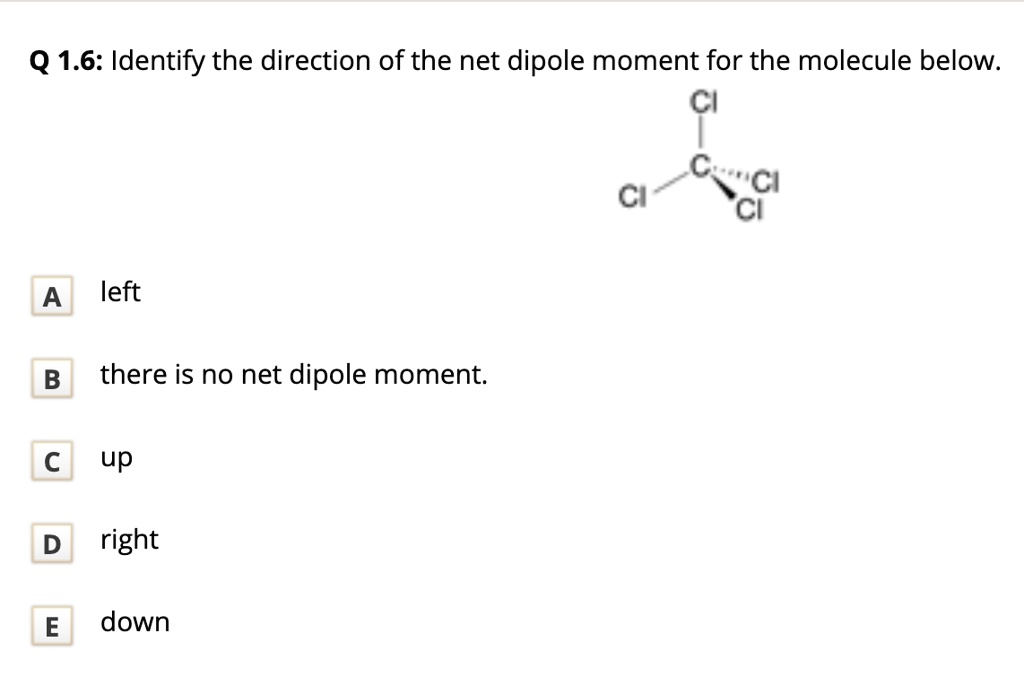
SOLVED Q 1.6 Identify the direction of the net dipole moment for the
Dipole moments occur when there is a separation of charge. A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moment (μ μ) is the measure of net molecular polarity, which is the.
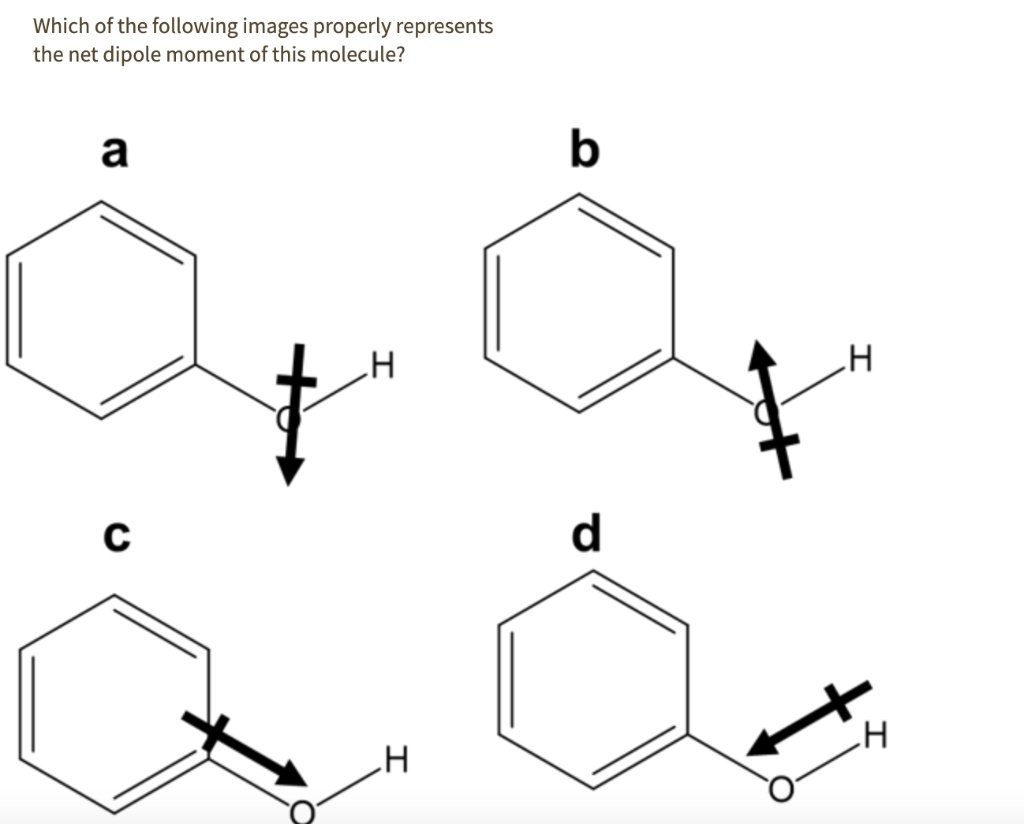
Which of the following images properly represents the net dipole moment
A molecule possesses a net dipole moment when polar covalent bonds create an. Dipole moment (μ μ) is the measure of net molecular polarity, which is the. Dipole moments occur when there is a separation of charge. A net dipole moment in a molecule exists when there is an uneven distribution of electron.
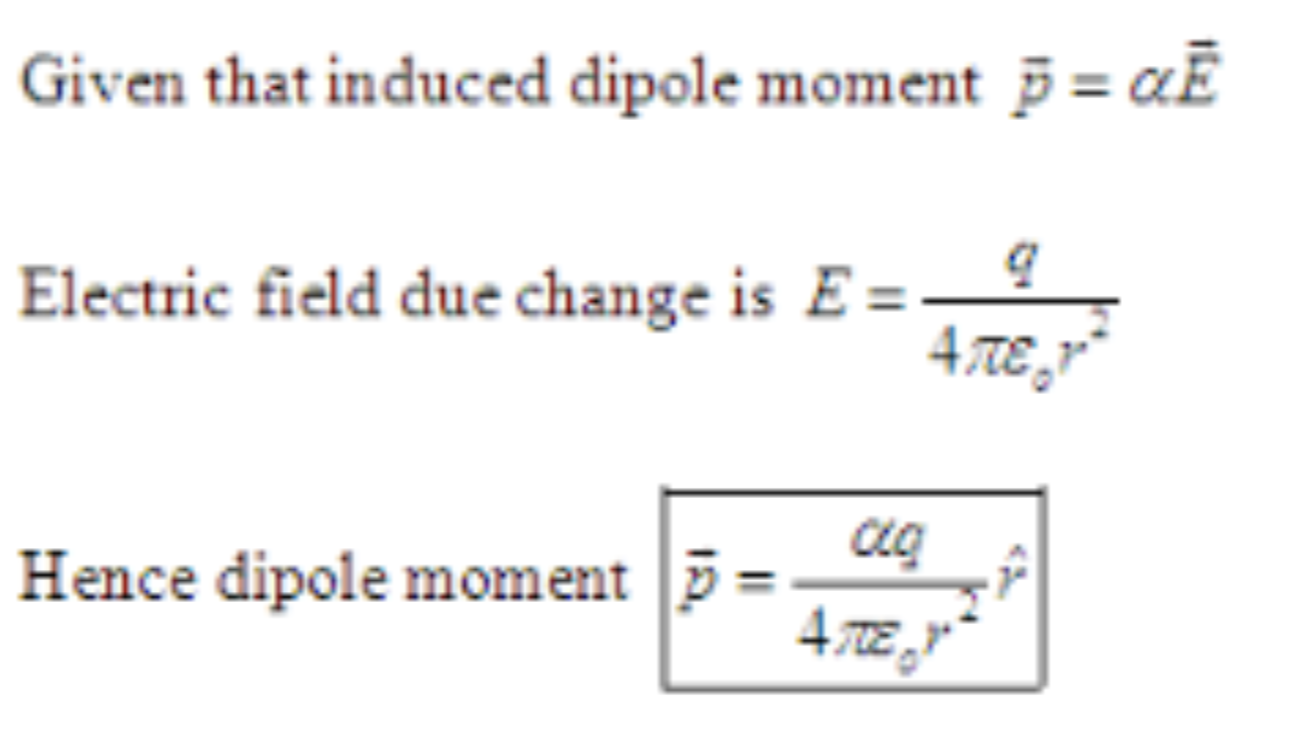
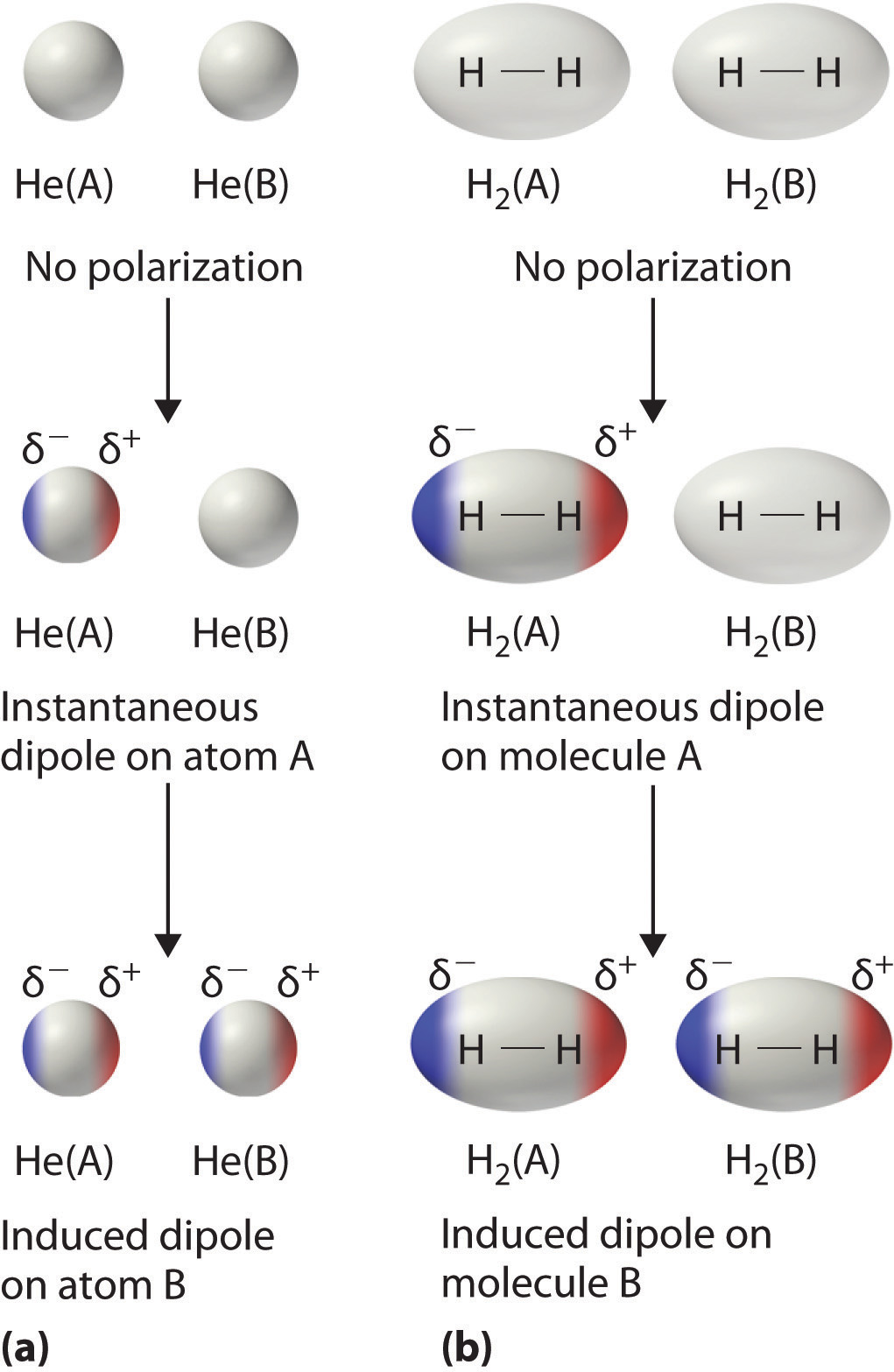
Solved An electric field can induce an electric dipole in a
Dipole moment (μ μ) is the measure of net molecular polarity, which is the. A net dipole moment in a molecule exists when there is an uneven distribution of electron. A molecule possesses a net dipole moment when polar covalent bonds create an. Dipole moments occur when there is a separation of charge.
What Causes Dipole Dipole Forces
Dipole moment (μ μ) is the measure of net molecular polarity, which is the. Dipole moments occur when there is a separation of charge. A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron.
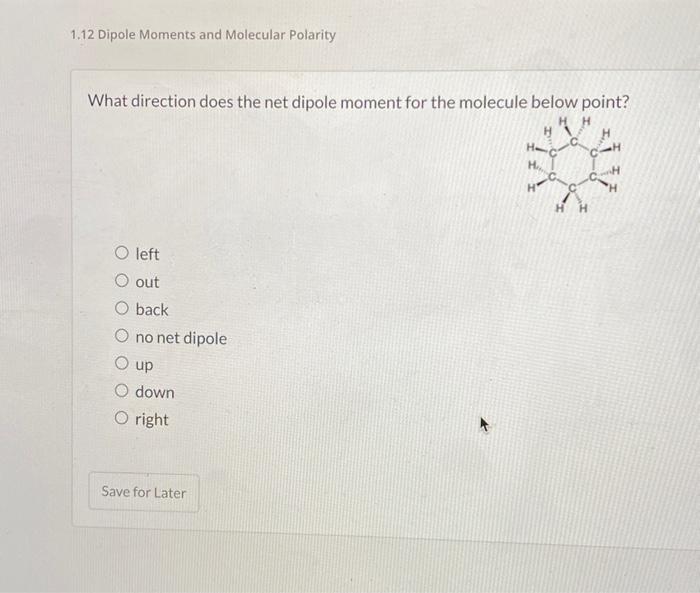
Solved 1.12 Dipole Moments and Molecular Polarity What
A molecule possesses a net dipole moment when polar covalent bonds create an. Dipole moment (μ μ) is the measure of net molecular polarity, which is the. A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge.
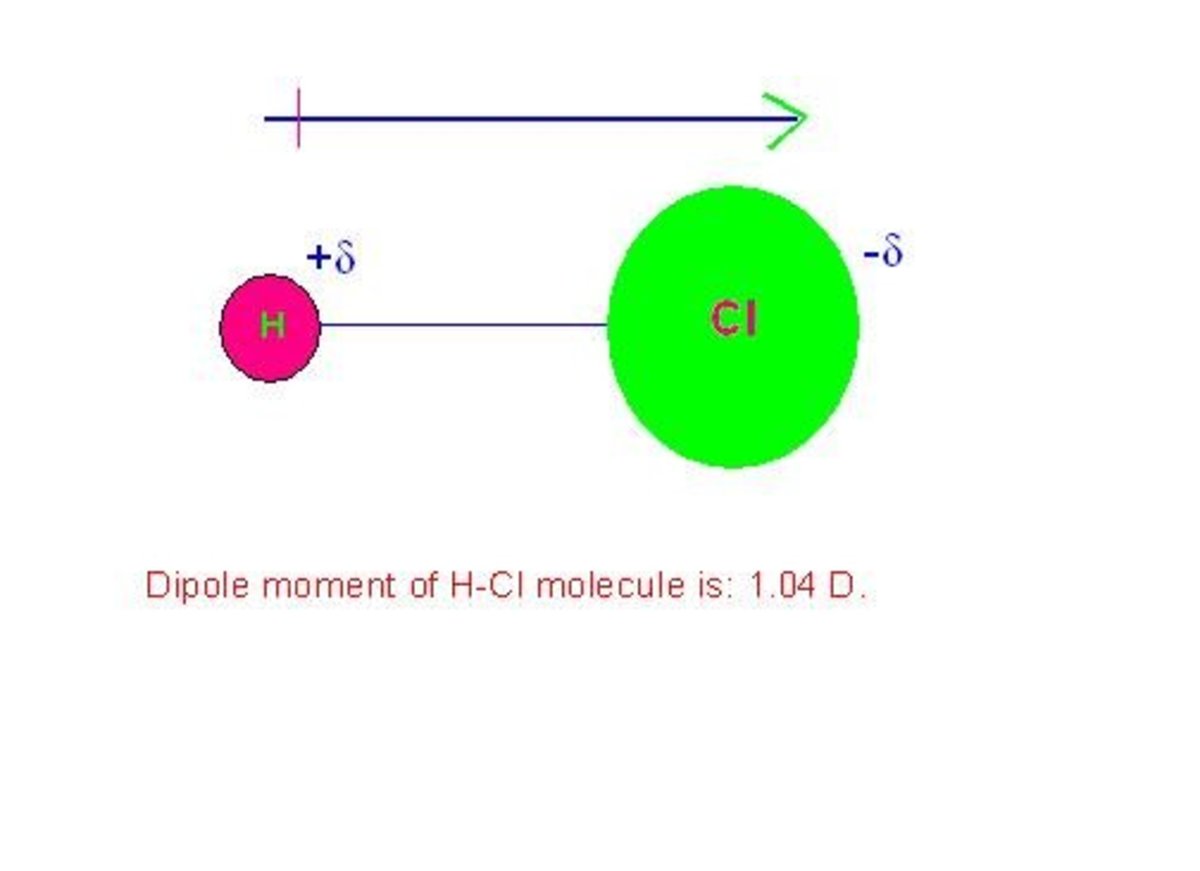
Permanent Dipole Moment
A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge. Dipole moment (μ μ) is the measure of net molecular polarity, which is the.
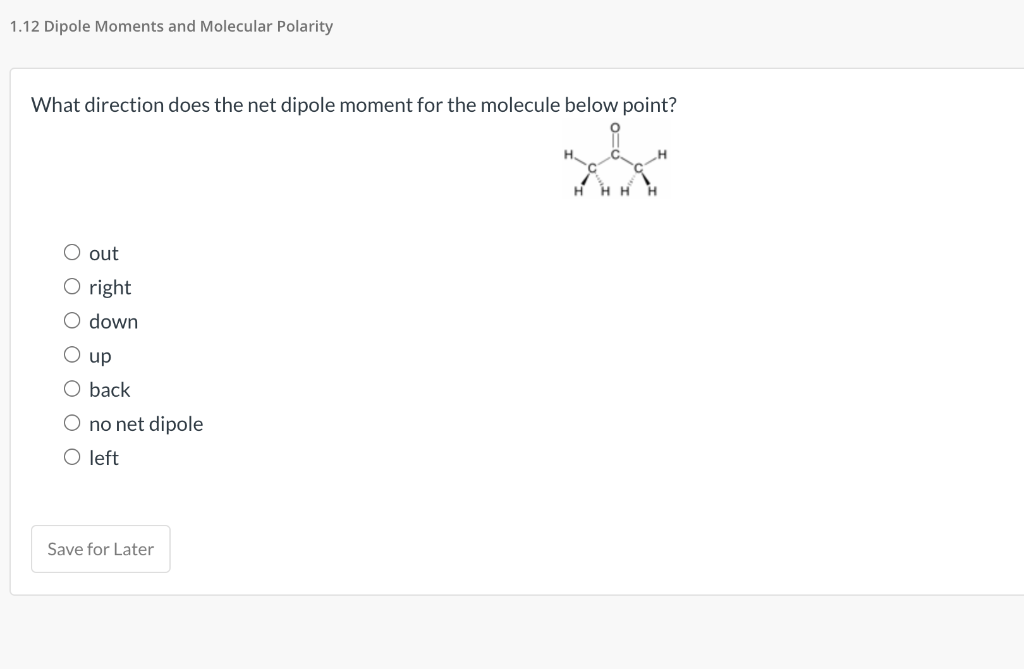
Solved What direction does the net dipole moment for the
Dipole moment (μ μ) is the measure of net molecular polarity, which is the. A net dipole moment in a molecule exists when there is an uneven distribution of electron. A molecule possesses a net dipole moment when polar covalent bonds create an. Dipole moments occur when there is a separation of charge.
Lucid Understanding Of, “Dipole Moment Of A Molecule” HubPages
A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge. A molecule possesses a net dipole moment when polar covalent bonds create an. Dipole moment (μ μ) is the measure of net molecular polarity, which is the.
Dipole Moment (Μ Μ) Is The Measure Of Net Molecular Polarity, Which Is The.
A molecule possesses a net dipole moment when polar covalent bonds create an. A net dipole moment in a molecule exists when there is an uneven distribution of electron. Dipole moments occur when there is a separation of charge.