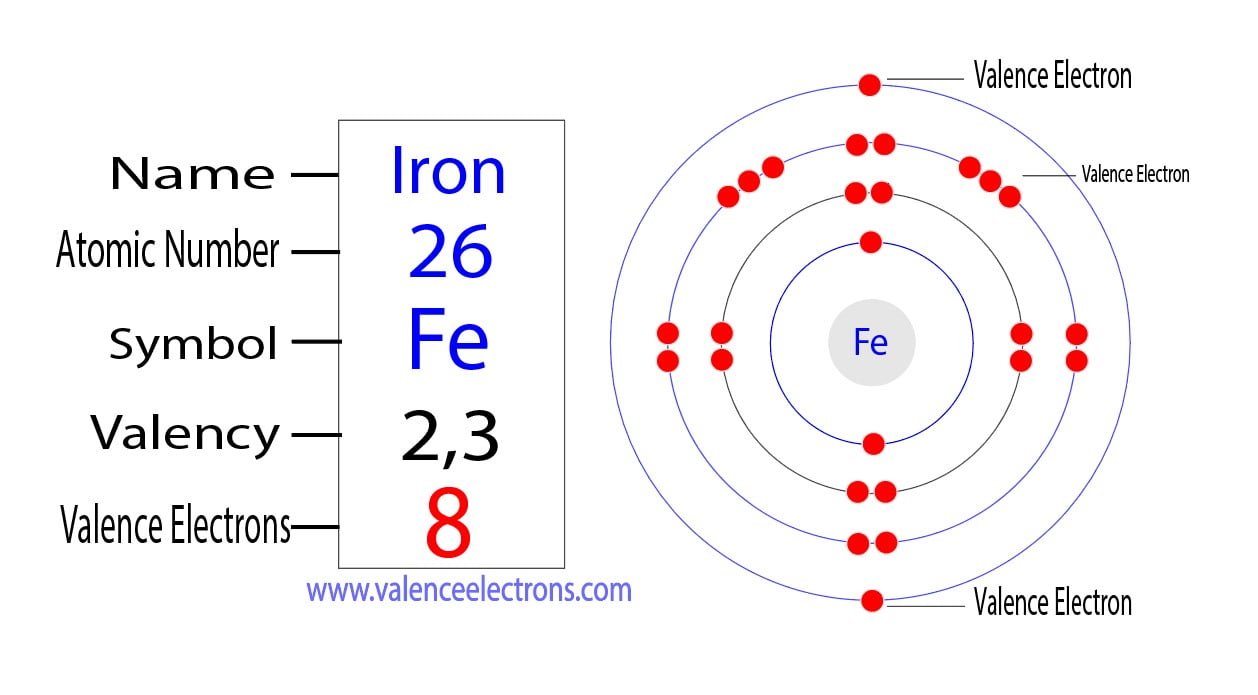
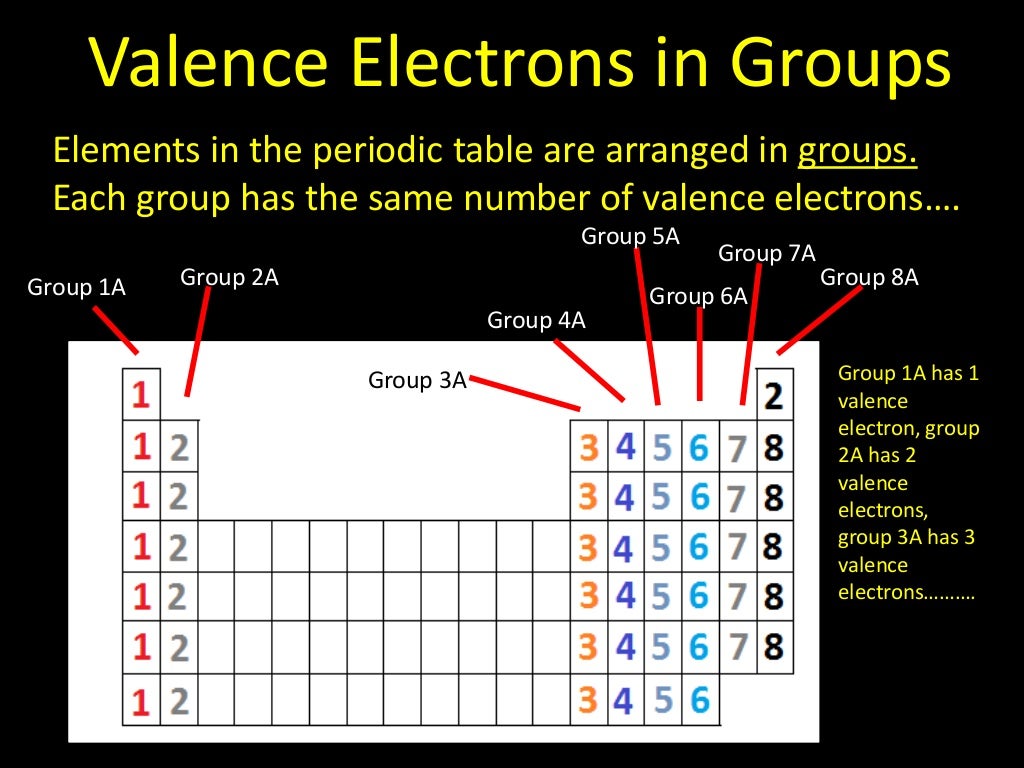
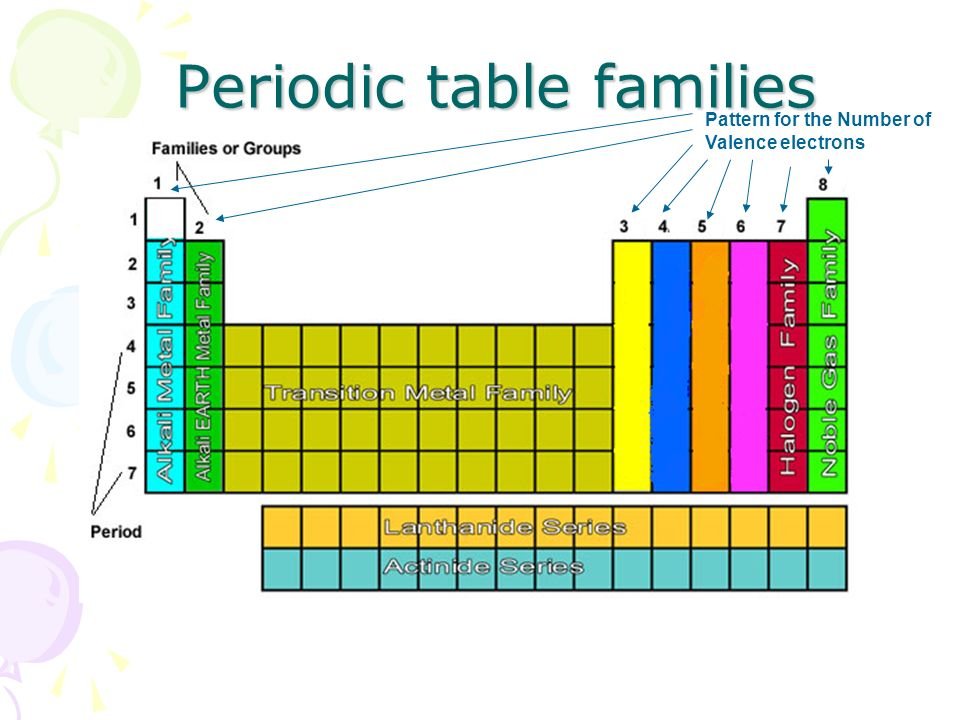
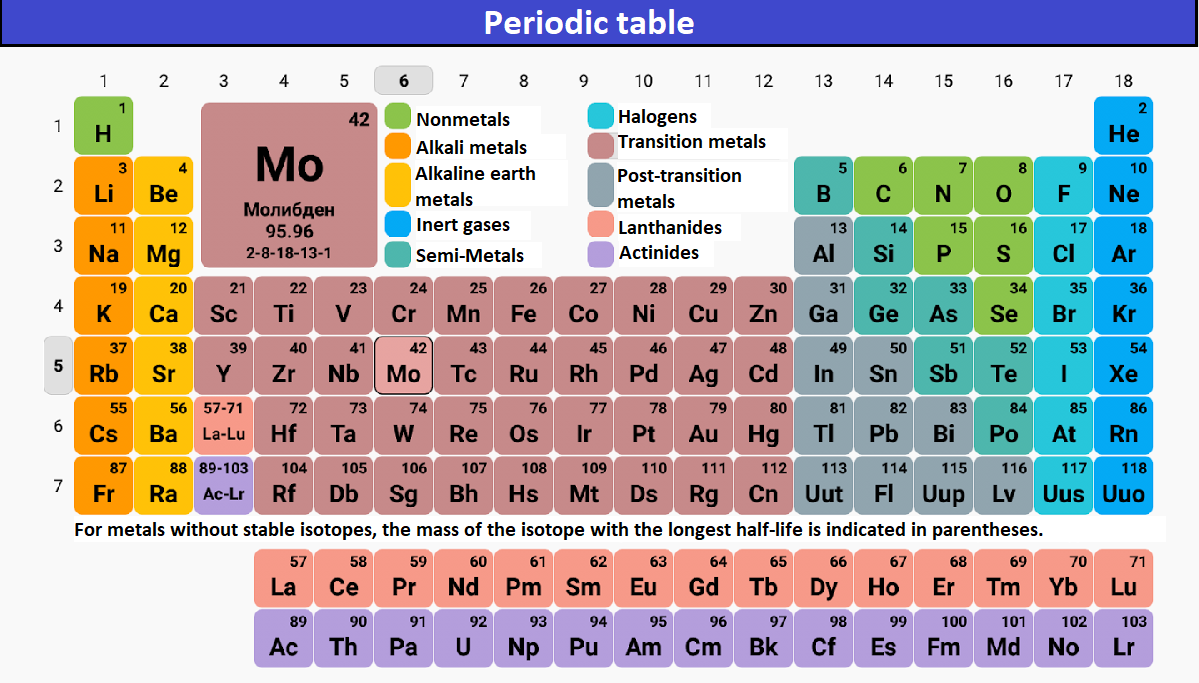
What Element Has 3 Valence Electrons - Elements of group iiia have three valence electrons: The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are boron (b) and aluminum (al).
The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons: The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The element that has 3 valence electrons in the 3rd energy level is aluminum.
The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The elements that have 3 valence electrons are boron (b) and aluminum (al). The element that has 3 valence electrons in the 3rd energy level is aluminum. Elements of group iiia have three valence electrons:
Valence electrons periodic table ladergadget
The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons: The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are nitrogen, phosphorus, and boron.
How to Find the Valence Electrons for Phosphorus (P)?
The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons: The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are nitrogen, phosphorus, and boron.
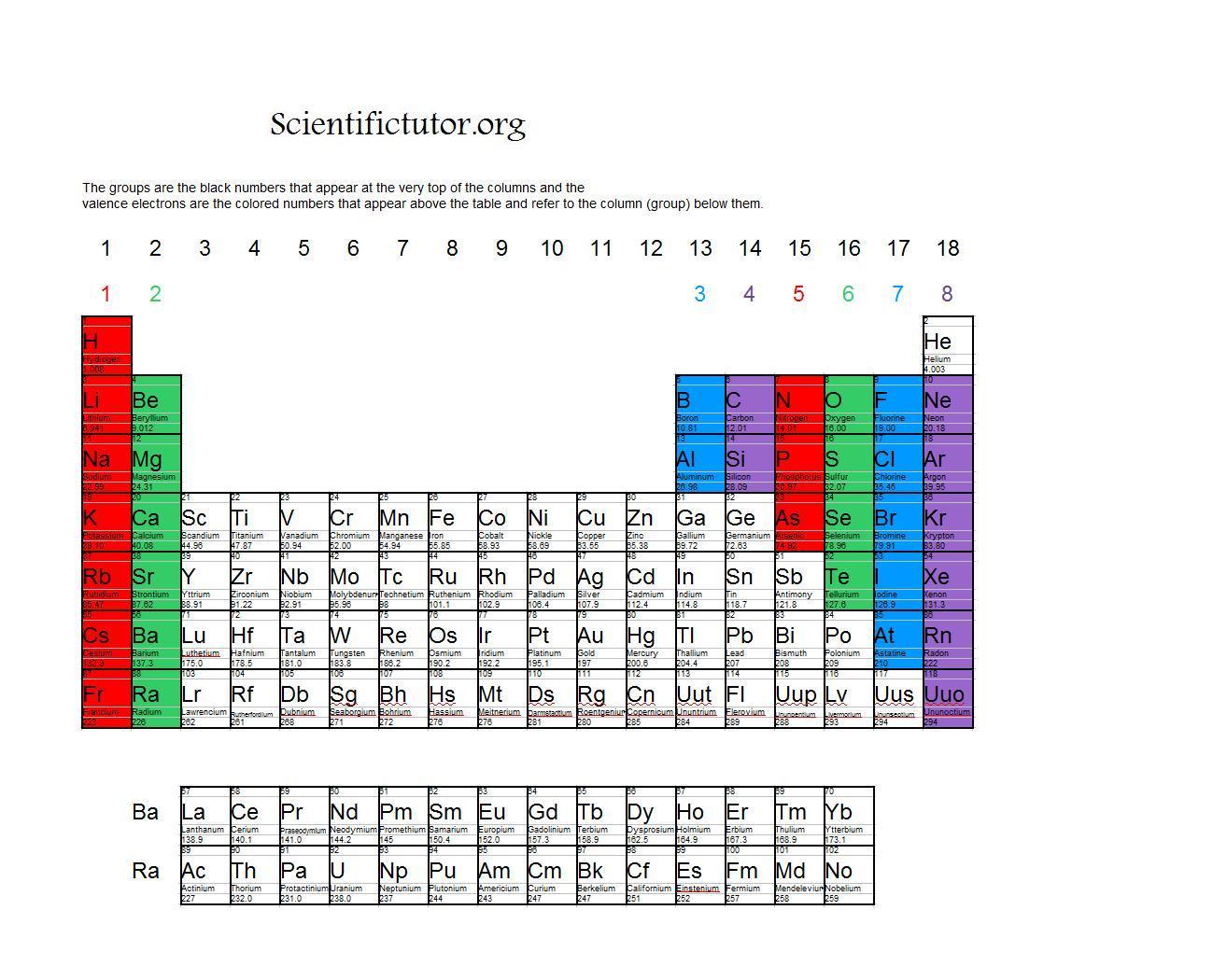
Periodic Table Valence Electrons
The elements that have 3 valence electrons are boron (b) and aluminum (al). The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. Elements of group iiia have three valence electrons: The element that has 3 valence electrons in the 3rd energy level is aluminum.
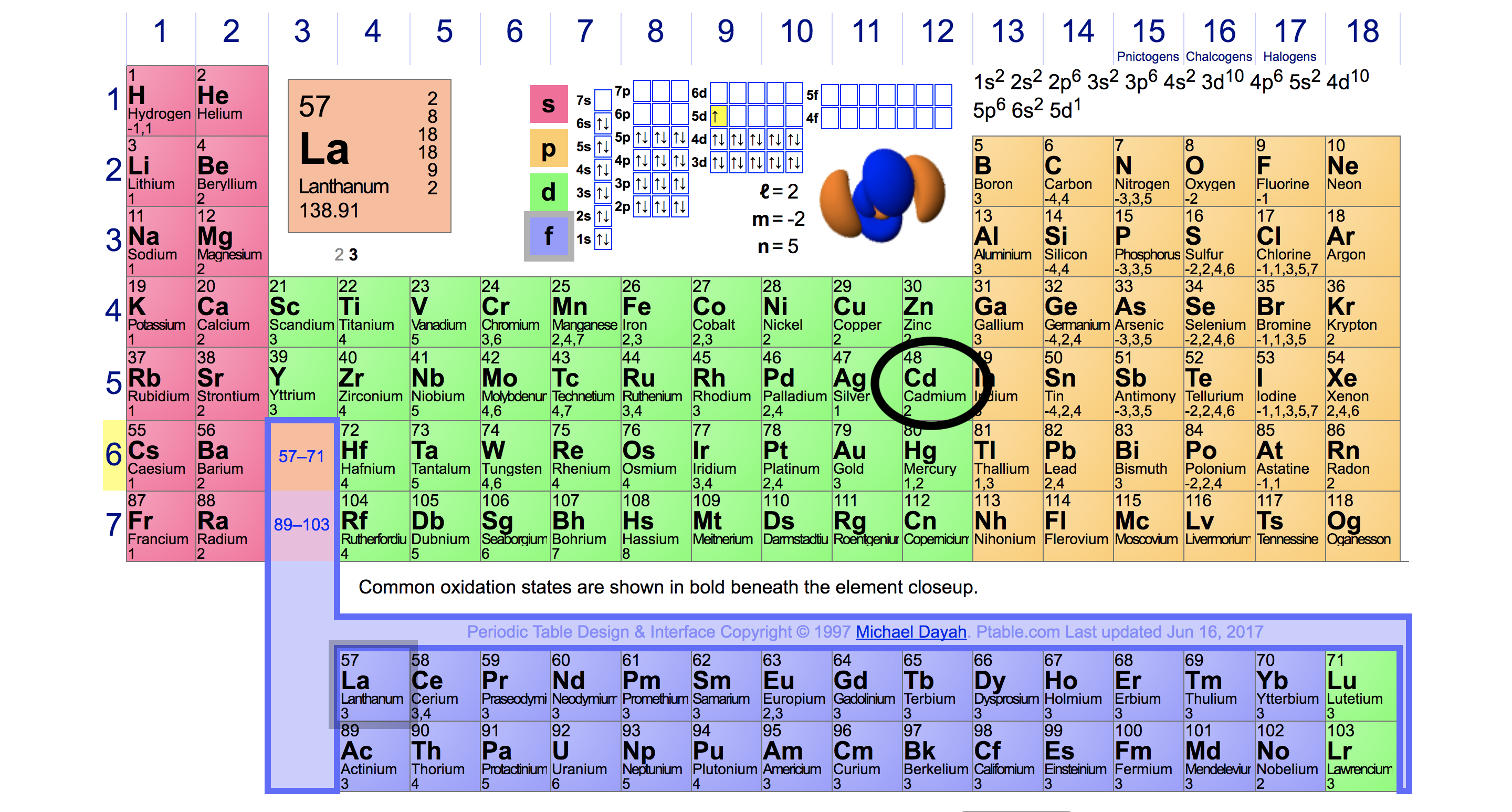
An element has 3 valence electrons in 3rd shell Name the element and
The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. Elements of group iiia have three valence electrons: The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are boron (b) and aluminum (al).
Periodic Table Valence Electrons
The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons:
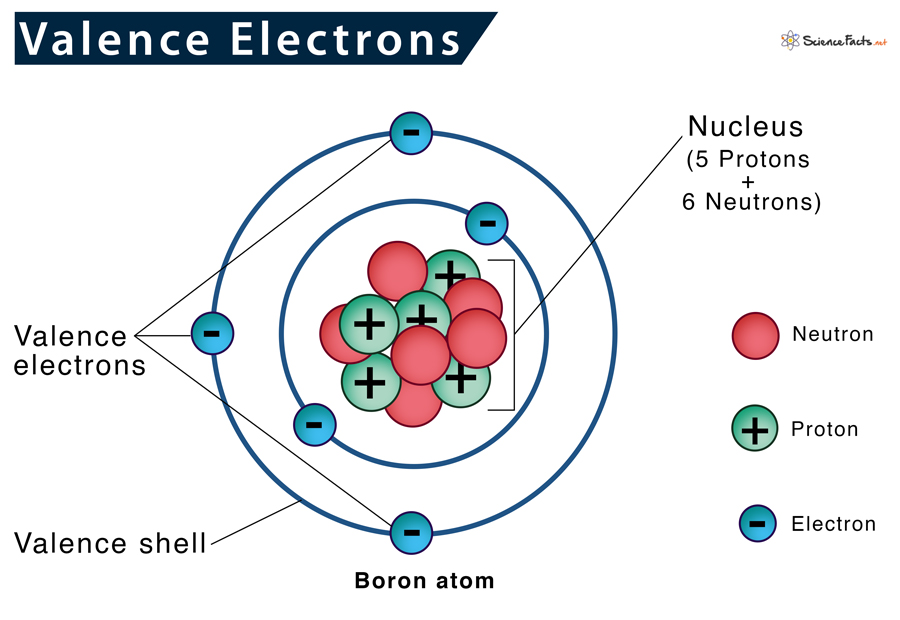
Valence Electrons Definition, Configuration, Examples
The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons: The elements that have 3 valence electrons are nitrogen, phosphorus, and boron.
Periodic Table Valence Electrons
The elements that have 3 valence electrons are boron (b) and aluminum (al). The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The element that has 3 valence electrons in the 3rd energy level is aluminum. Elements of group iiia have three valence electrons:
Valence Electrons Definition, Location, Importance, and Diagram
The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons:
Chem Valence Electrons Scientific Tutor
The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are boron (b) and aluminum (al). The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. Elements of group iiia have three valence electrons:
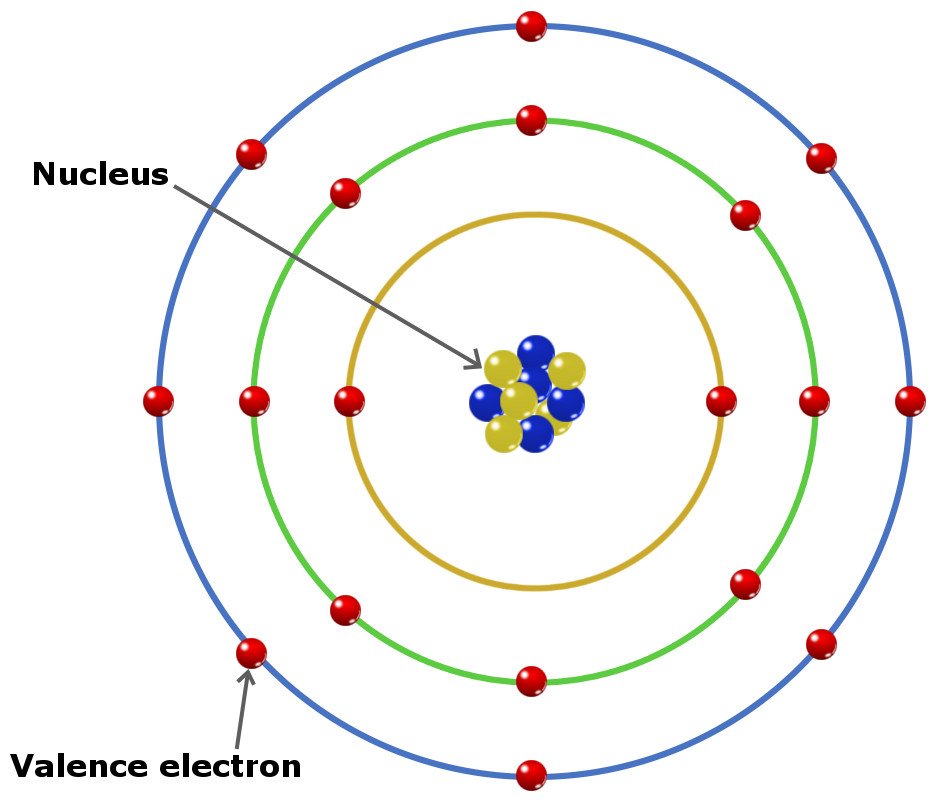
Valence Electrons — Definition & Importance Expii
Elements of group iiia have three valence electrons: The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The element that has 3 valence electrons in the 3rd energy level is aluminum. The elements that have 3 valence electrons are boron (b) and aluminum (al).
The Element That Has 3 Valence Electrons In The 3Rd Energy Level Is Aluminum.
The elements that have 3 valence electrons are nitrogen, phosphorus, and boron. The elements that have 3 valence electrons are boron (b) and aluminum (al). Elements of group iiia have three valence electrons: