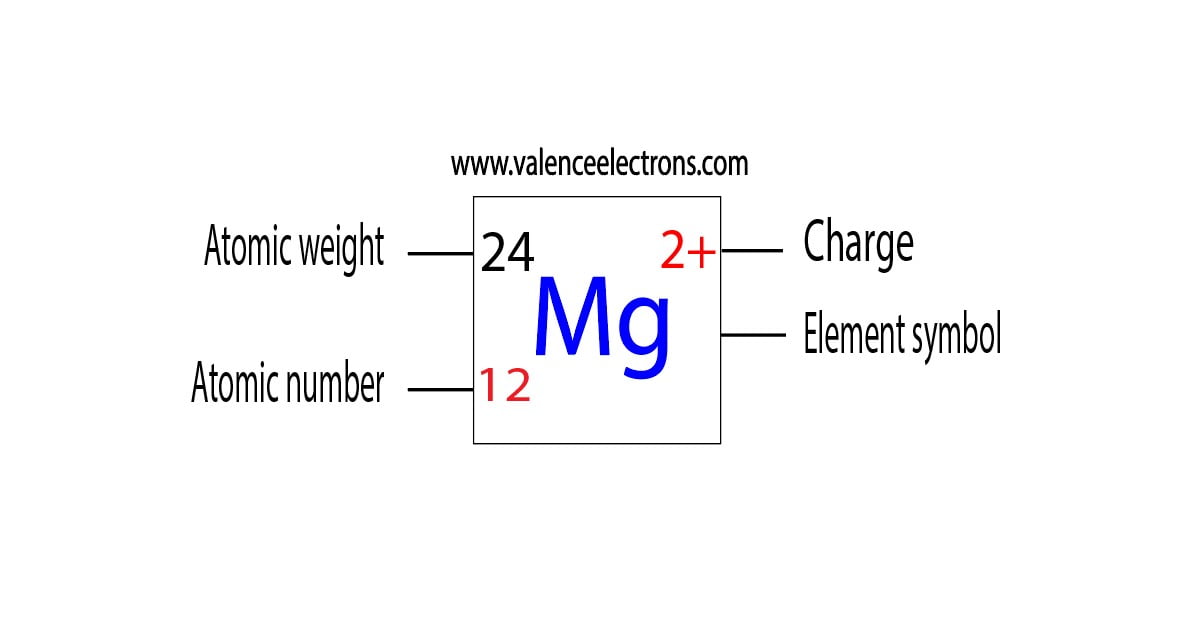
What Happens When A Magnesium Atom Loses An Electron - What happens when a magnesium atom loses an electron? When a magnesium atom (mg) forms an ionic bond and reaches the electron. Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation.
What happens when a magnesium atom loses an electron? Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation. When a magnesium atom (mg) forms an ionic bond and reaches the electron.
What happens when a magnesium atom loses an electron? When a magnesium atom (mg) forms an ionic bond and reaches the electron. Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation.
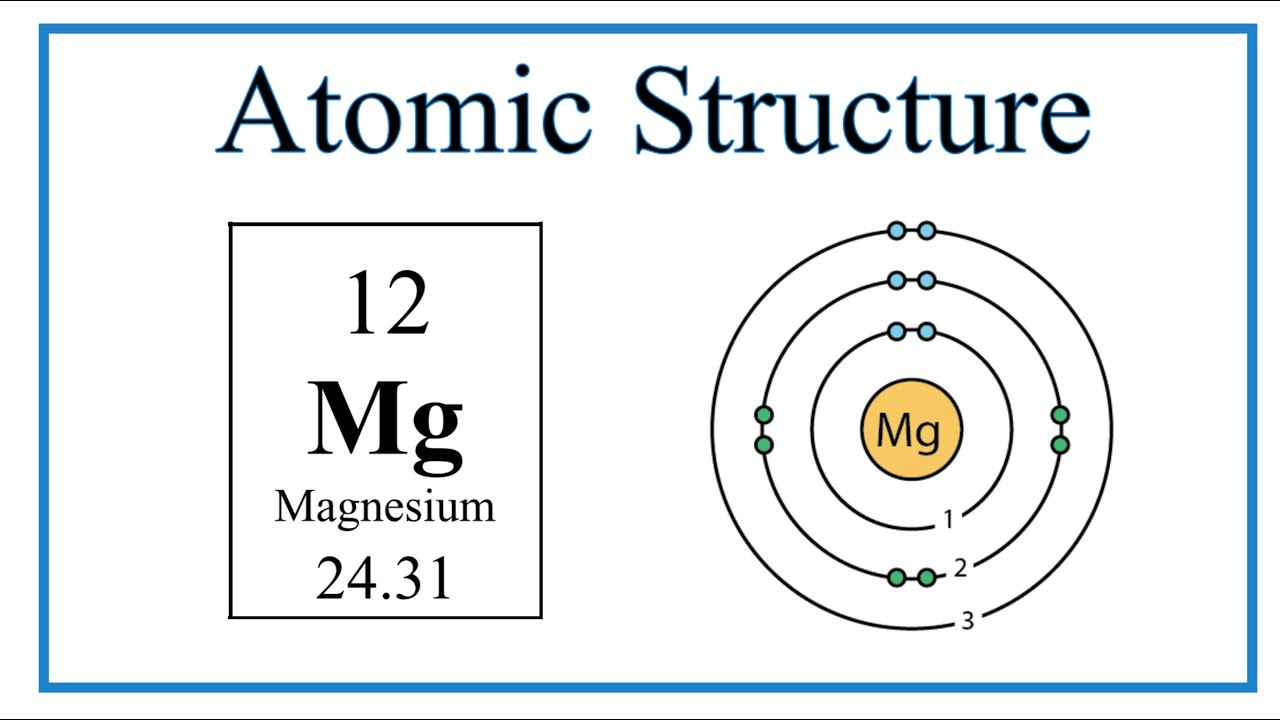
How to Write the Electron Configuration for Magnesium (Mg)
When magnesium (mg) loses 2 electrons, it undergoes oxidation. What happens when a magnesium atom loses an electron? Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron.
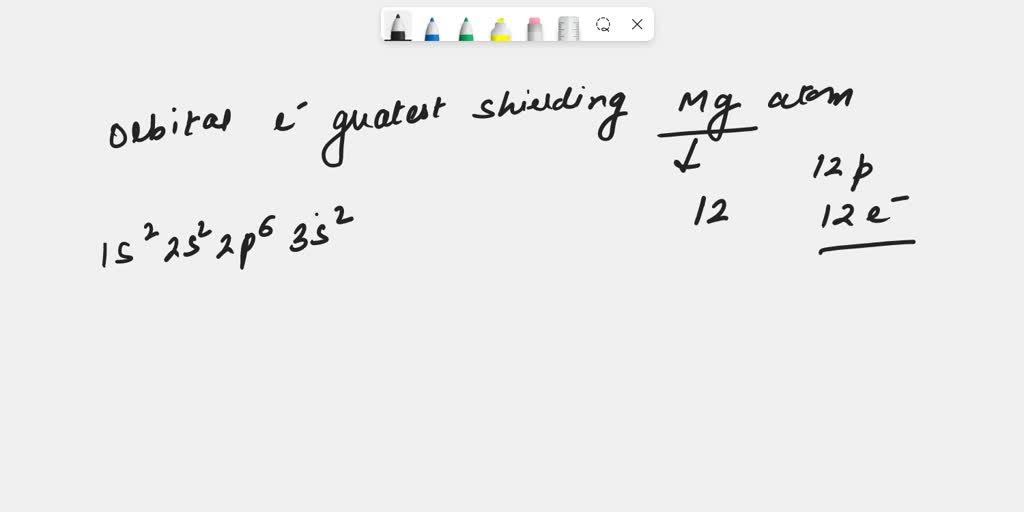
SOLVED In which orbital does an electron in a magnesium atom
When a magnesium atom (mg) forms an ionic bond and reaches the electron. What happens when a magnesium atom loses an electron? When magnesium (mg) loses 2 electrons, it undergoes oxidation. Each magnesium atom loses 2 electrons and each iodine atom gains an electron.
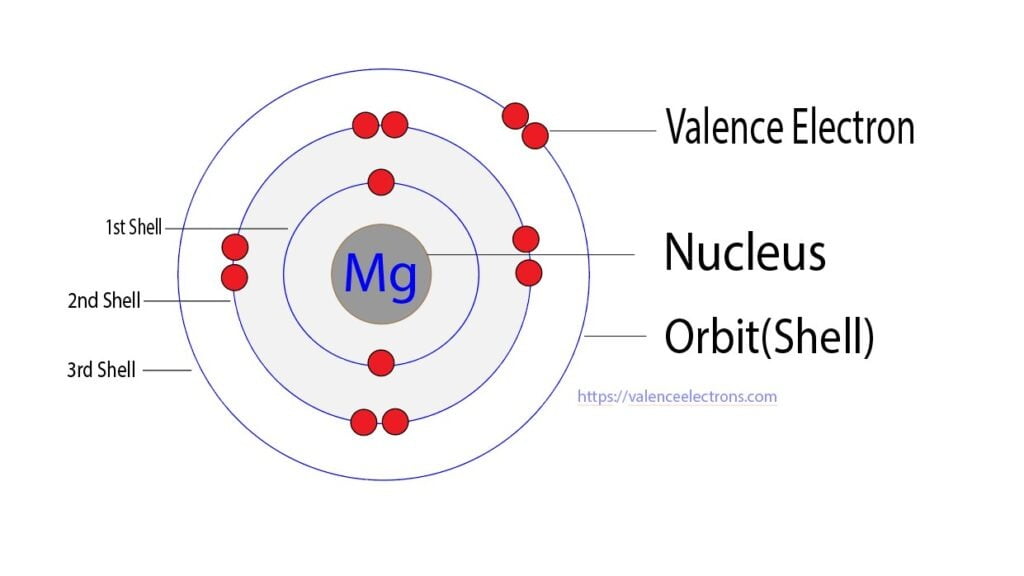
Electron Arrangement of Magnesium
What happens when a magnesium atom loses an electron? When magnesium (mg) loses 2 electrons, it undergoes oxidation. Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron.
When an Atom Loses an Electron What Is Its Charge What is the charge
What happens when a magnesium atom loses an electron? When magnesium (mg) loses 2 electrons, it undergoes oxidation. Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron.
Magnesium Electron Configuration
Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation. What happens when a magnesium atom loses an electron?
Magnesium Electron Configuration
Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation. What happens when a magnesium atom loses an electron? When a magnesium atom (mg) forms an ionic bond and reaches the electron.
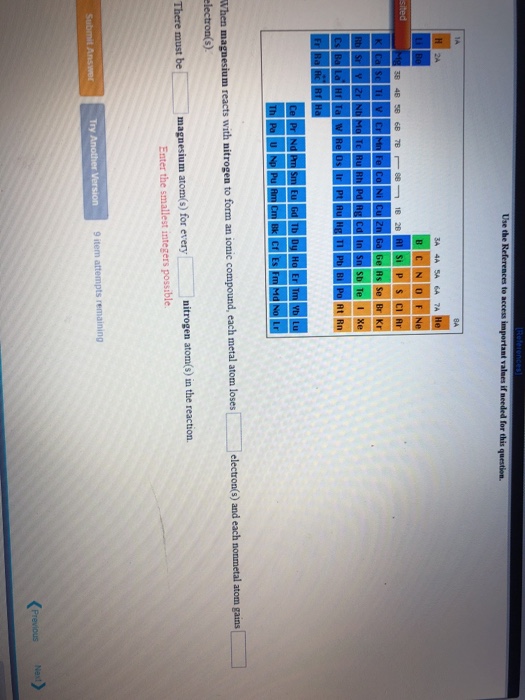
Solved 1. When magnesium reacts with nitrogen to form an
Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron. What happens when a magnesium atom loses an electron? When magnesium (mg) loses 2 electrons, it undergoes oxidation.
How to Write the Electron Configuration for Magnesium (Mg)
Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron. What happens when a magnesium atom loses an electron? When magnesium (mg) loses 2 electrons, it undergoes oxidation.
SOLVED A magnesium atom that loses 2 electrons a A) Positive
When a magnesium atom (mg) forms an ionic bond and reaches the electron. What happens when a magnesium atom loses an electron? Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation.
SOLVED Read the following chemical equation Mg(s) + H+ + Cl → Mg2
When a magnesium atom (mg) forms an ionic bond and reaches the electron. What happens when a magnesium atom loses an electron? Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When magnesium (mg) loses 2 electrons, it undergoes oxidation.
When Magnesium (Mg) Loses 2 Electrons, It Undergoes Oxidation.
Each magnesium atom loses 2 electrons and each iodine atom gains an electron. When a magnesium atom (mg) forms an ionic bond and reaches the electron. What happens when a magnesium atom loses an electron?