What Happens When Sodium Reacts With Chlorine - The sodium atom loses an electron to become a positive ion. This is an example of ionic bonding. This electron is then gained by the chlorine atom which becomes a negative ion. Sodium and chloride form an ionic bond. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) As this happens, the electron is transferred from. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas.
When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. As this happens, the electron is transferred from. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. This electron is then gained by the chlorine atom which becomes a negative ion. This is an example of ionic bonding. The sodium atom loses an electron to become a positive ion. Sodium and chloride form an ionic bond.
When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. This is an example of ionic bonding. As this happens, the electron is transferred from. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. This electron is then gained by the chlorine atom which becomes a negative ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) The sodium atom loses an electron to become a positive ion. Sodium and chloride form an ionic bond.
Solved 1. Sodium metal reacts violently with chlorine gas to
This electron is then gained by the chlorine atom which becomes a negative ion. As this happens, the electron is transferred from. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) This is an example of ionic bonding.
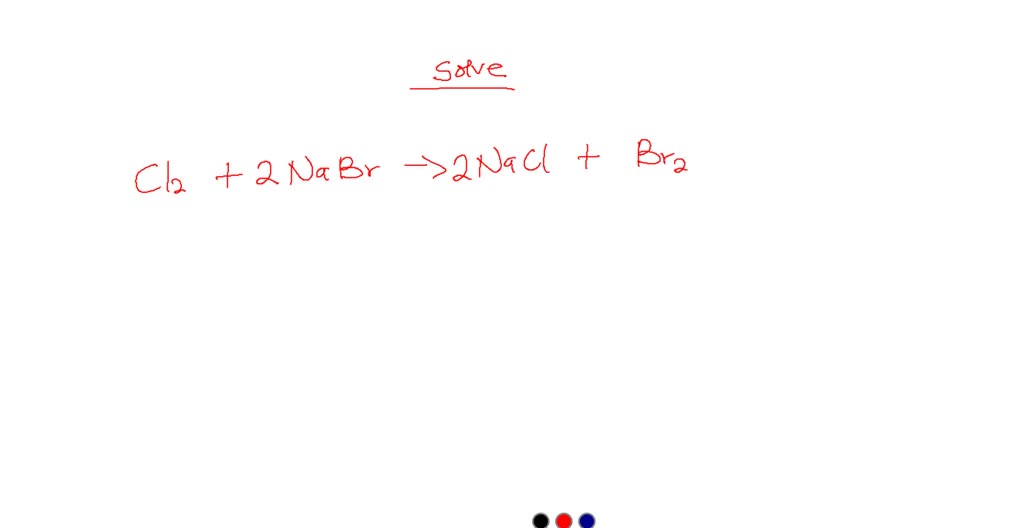
When chlorine gas is bubbled into a solution of sodium bromide, the
This electron is then gained by the chlorine atom which becomes a negative ion. The sodium atom loses an electron to become a positive ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) Sodium and chloride form an ionic bond. This is an example of ionic bonding.
SOLVEDDraw a diagram to show what happens when a sodium atom reacts
Sodium and chloride form an ionic bond. The sodium atom loses an electron to become a positive ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. This is an example of ionic bonding. This electron is then gained by the chlorine atom which becomes a negative ion.
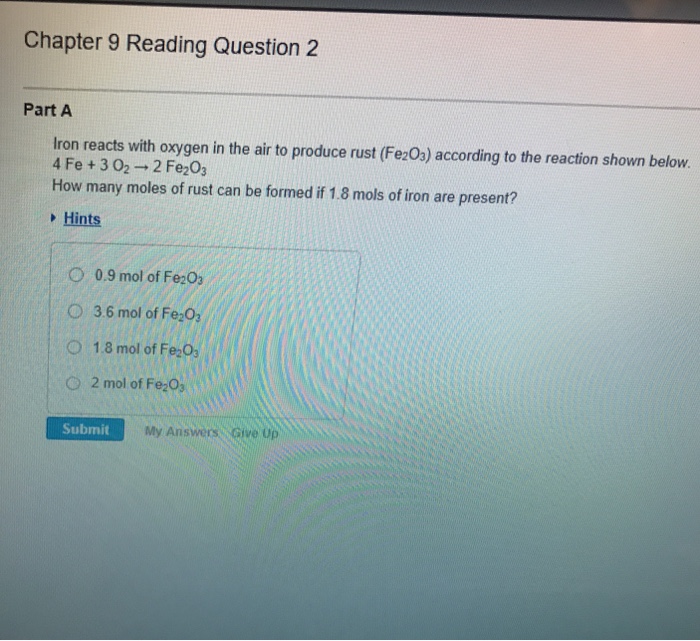
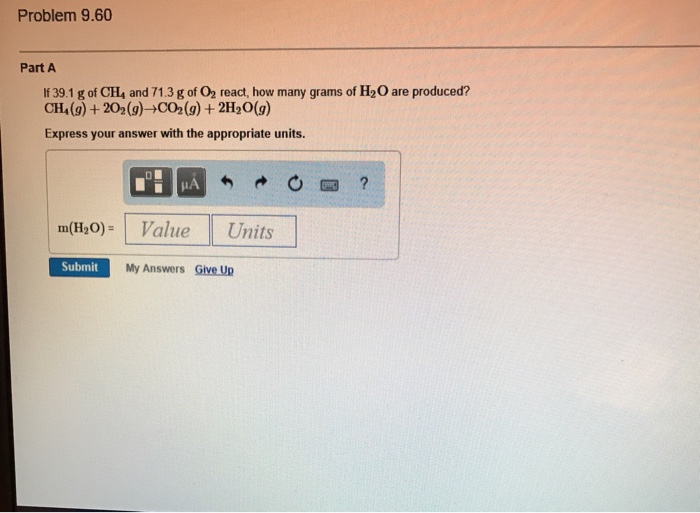
Solved Part A A sample of sodium reacts completely with
As this happens, the electron is transferred from. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) This is an example of ionic bonding. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. Sodium and chloride form an ionic bond.
Chlorine reacts with sodium to form the compound NaCl. Chlorine also reac..
This is an example of ionic bonding. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) As this happens, the electron is transferred from. Sodium and chloride form an ionic bond. The sodium atom loses an electron to become a positive ion.
Solved Part A A sample of sodium reacts completely with
👍 helpful ( 0 ) 👎 not helpful ( 0 ) This electron is then gained by the chlorine atom which becomes a negative ion. The sodium atom loses an electron to become a positive ion. As this happens, the electron is transferred from. Sodium and chloride form an ionic bond.
SOLVED What happens when (4)a. Magnesium reacts with dilute HCl?b
This electron is then gained by the chlorine atom which becomes a negative ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) This is an example of ionic bonding. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. As.
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce
When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. This electron is then gained by the chlorine atom which becomes a negative ion. This is an example of ionic bonding. Therefore the sodium atom loses one electron from its outer shell and.
Solved Part A A sample of sodium reacts completely with
This electron is then gained by the chlorine atom which becomes a negative ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. As this happens, the electron is transferred from. This is an example of ionic bonding. 👍 helpful ( 0 ) 👎 not helpful ( 0 )
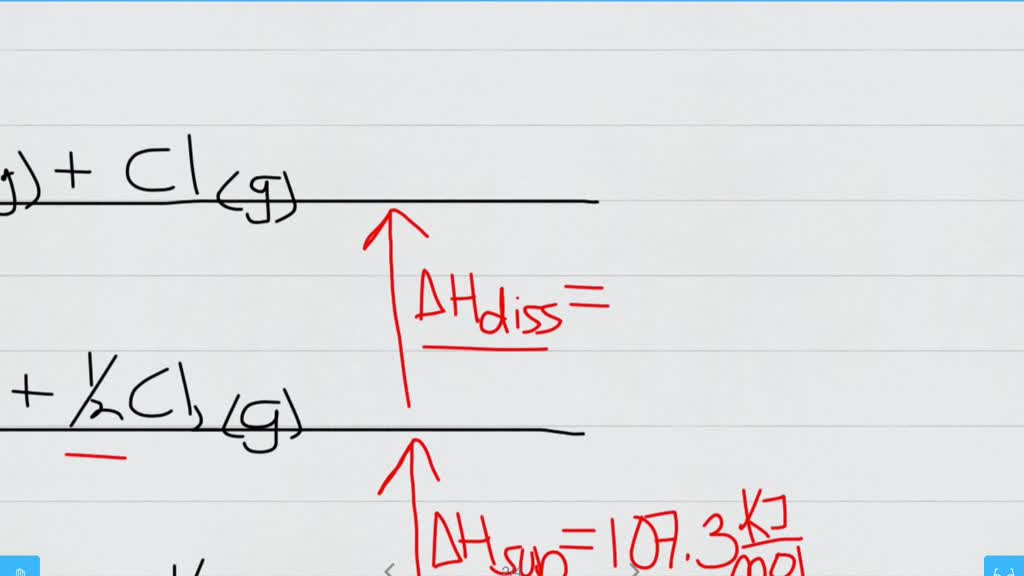
SOLVEDSodium reacts with chlorine gas according to the reaction 2 Na
This electron is then gained by the chlorine atom which becomes a negative ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. The sodium atom loses an electron to become.
As This Happens, The Electron Is Transferred From.
This is an example of ionic bonding. This electron is then gained by the chlorine atom which becomes a negative ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas.
Sodium And Chloride Form An Ionic Bond.
👍 helpful ( 0 ) 👎 not helpful ( 0 ) The sodium atom loses an electron to become a positive ion.