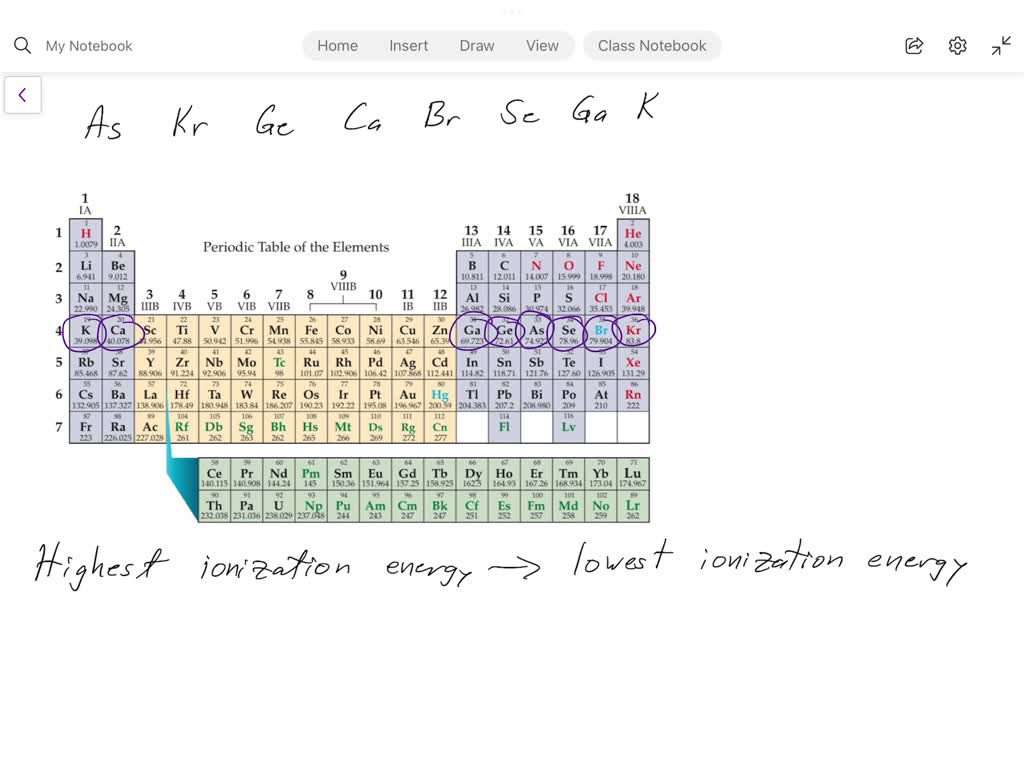
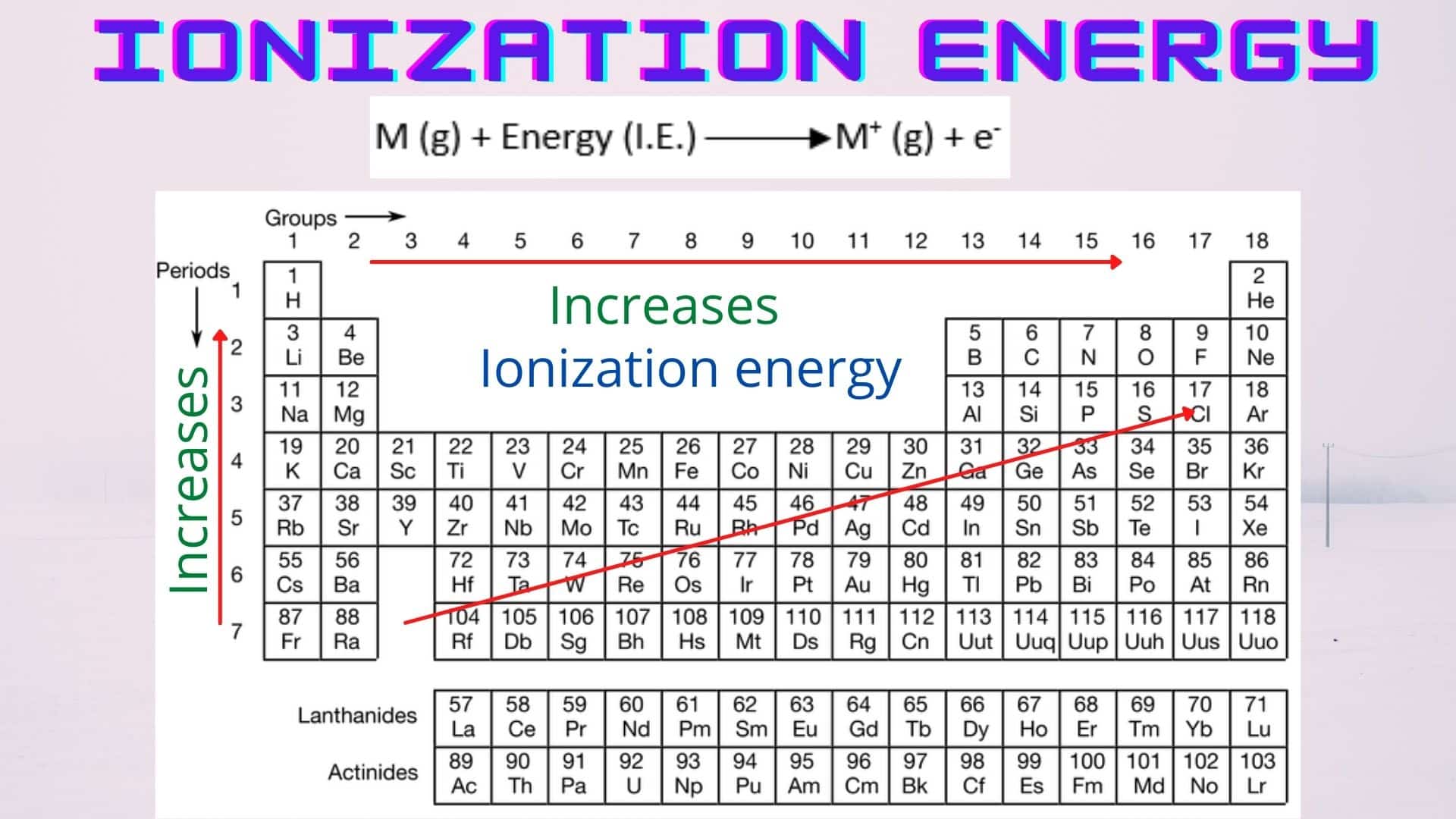
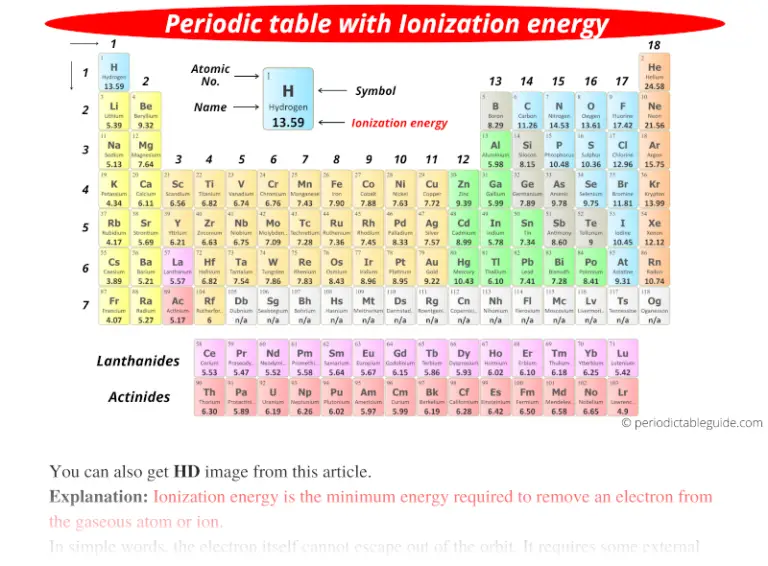
What Has The Lowest Ionization Energy - The element with the lowest ionization energy is cesium (cs). 119 rows for chemistry students and teachers: In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. The tabular chart on the right is arranged by. Cesium (cs) has the lowest ionization energy among the options provided. 120 rows ionization energy chart of all the elements is given below. Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. 104 rows to list the elements order by ionization energy, click on the table headers.
Cesium (cs) has the lowest ionization energy among the options provided. In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. 120 rows ionization energy chart of all the elements is given below. 104 rows to list the elements order by ionization energy, click on the table headers. 119 rows for chemistry students and teachers: The element with the lowest ionization energy is cesium (cs). Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. The tabular chart on the right is arranged by.
104 rows to list the elements order by ionization energy, click on the table headers. 119 rows for chemistry students and teachers: In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. The tabular chart on the right is arranged by. Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. 120 rows ionization energy chart of all the elements is given below. The element with the lowest ionization energy is cesium (cs). Cesium (cs) has the lowest ionization energy among the options provided.
Ionization energy Definition & Facts Britannica
In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. 120 rows ionization energy chart of all the elements is given below. 119 rows for chemistry students and teachers: Cesium (cs) has the lowest ionization energy among the options provided. Comparing these elements, cesium (cs) is an alkali metal at.
Ionization Energy Definition, Formulas, and Solved Examples
The element with the lowest ionization energy is cesium (cs). 120 rows ionization energy chart of all the elements is given below. Cesium (cs) has the lowest ionization energy among the options provided. 104 rows to list the elements order by ionization energy, click on the table headers. Comparing these elements, cesium (cs) is an alkali metal at the bottom.
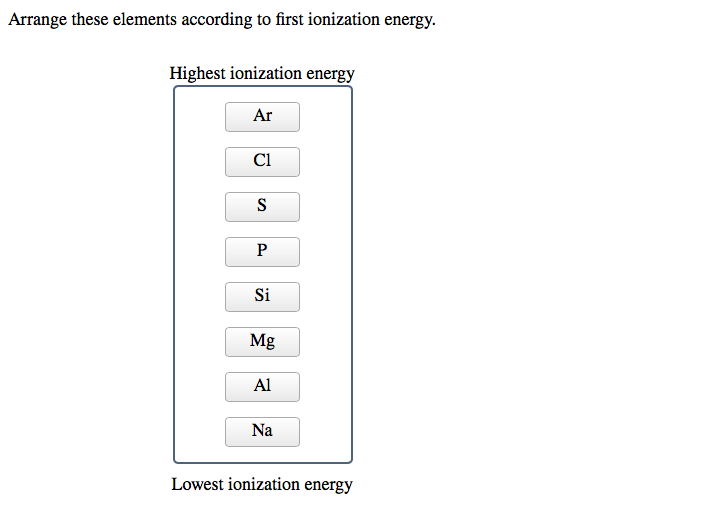
Solved Arrange these elements according to first ionization
Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. Cesium (cs) has the lowest ionization energy among the options provided. The element with the lowest ionization energy is cesium (cs). The tabular chart on the right is arranged by. 104 rows to list the elements order by ionization energy, click on the table.
Ionization energy Chemistry Steps
Cesium (cs) has the lowest ionization energy among the options provided. 104 rows to list the elements order by ionization energy, click on the table headers. 119 rows for chemistry students and teachers: The tabular chart on the right is arranged by. 120 rows ionization energy chart of all the elements is given below.
Ionization Energy Periodic Table Values Matttroy
Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. 119 rows for chemistry students and teachers: Cesium (cs) has the lowest ionization energy among the options provided. The element with the lowest ionization energy is cesium (cs). 120 rows ionization energy chart of all the elements is given below.
SOLVED 'Rank these elements according to first ionization energy
The tabular chart on the right is arranged by. In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. Cesium (cs) has the lowest ionization energy among the options provided. 120 rows ionization energy chart of all the elements is given below. Comparing these elements, cesium (cs) is an alkali.
Ionization energy and ionization potential Chemistry Notes
The element with the lowest ionization energy is cesium (cs). 120 rows ionization energy chart of all the elements is given below. In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. The tabular chart on the right is arranged by. Cesium (cs) has the lowest ionization energy among the.
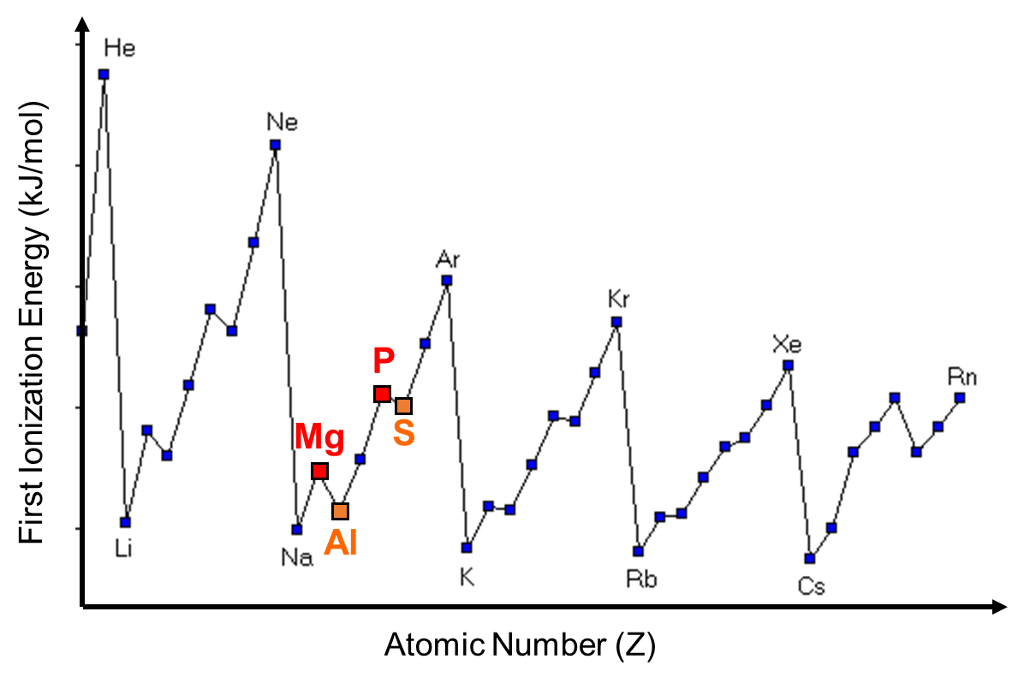
Ionization energy Group i Chemistry(iiiescuro)
In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. 104 rows to list the elements order by ionization energy, click on the table headers. 119 rows for chemistry students and teachers: Cesium (cs) has the lowest ionization energy among the options provided. The element with the lowest ionization energy.
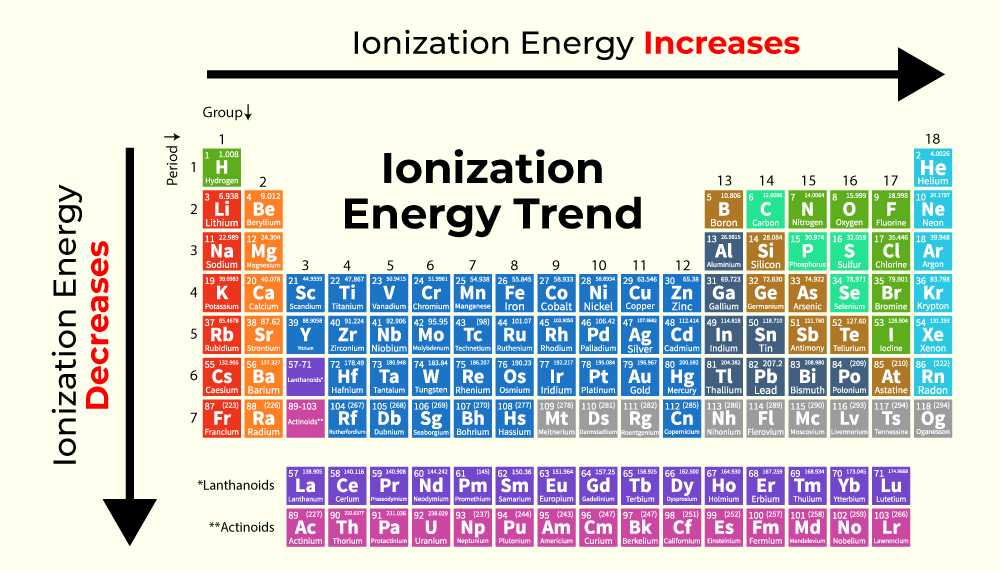
Periodic table with Ionization Energy Values (Labeled Image)
In the periodic table, fluorine f is in group 17, oxygen o and sulfur s are in group 16, and. Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. 104 rows to list the elements order by ionization energy, click on the table headers. The element with the lowest ionization energy is cesium.
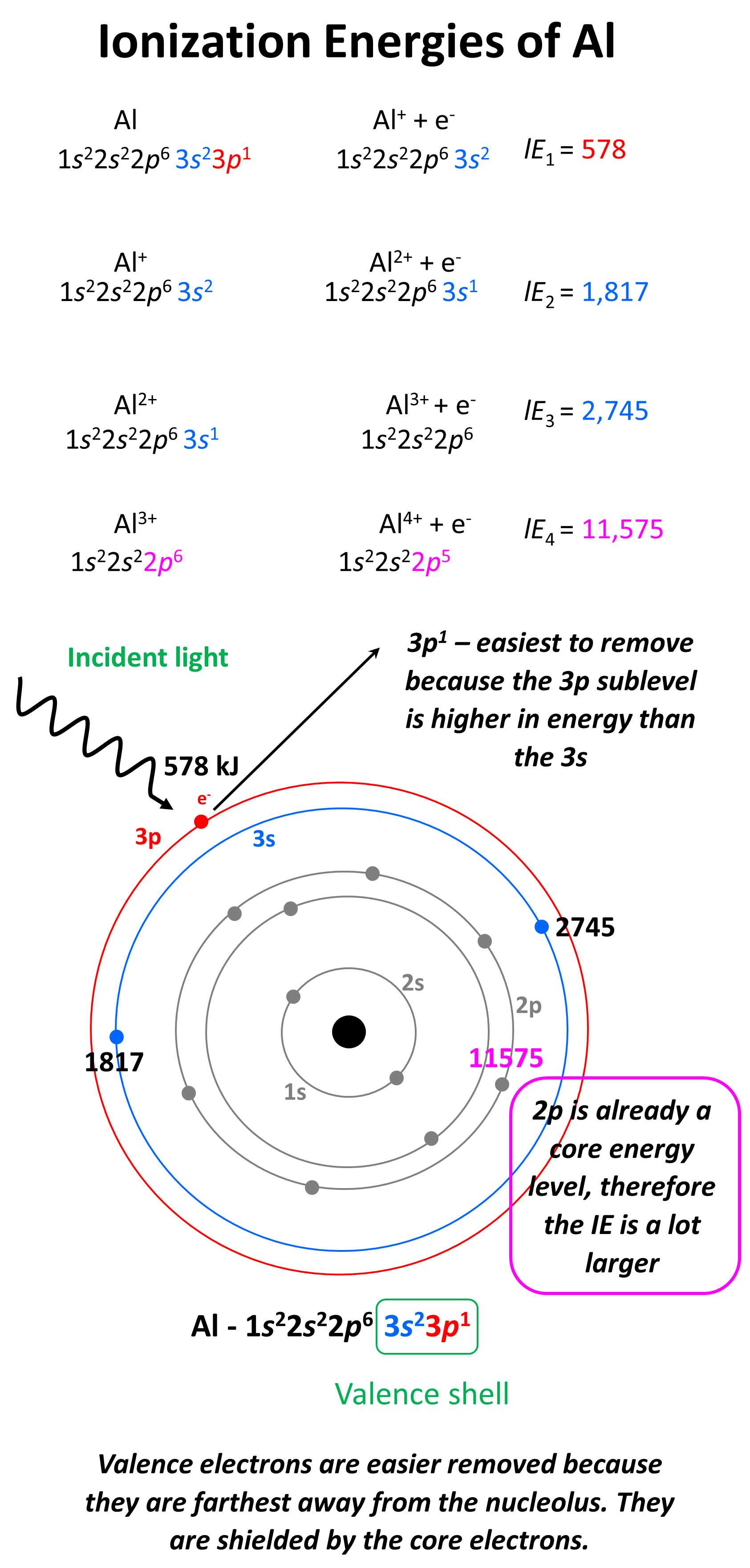
Ionization Energy the amount of energy required to remove an electron
Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known. 119 rows for chemistry students and teachers: 120 rows ionization energy chart of all the elements is given below. The tabular chart on the right is arranged by. The element with the lowest ionization energy is cesium (cs).
104 Rows To List The Elements Order By Ionization Energy, Click On The Table Headers.
The tabular chart on the right is arranged by. The element with the lowest ionization energy is cesium (cs). Cesium (cs) has the lowest ionization energy among the options provided. Comparing these elements, cesium (cs) is an alkali metal at the bottom of its group, known.
In The Periodic Table, Fluorine F Is In Group 17, Oxygen O And Sulfur S Are In Group 16, And.
120 rows ionization energy chart of all the elements is given below. 119 rows for chemistry students and teachers: