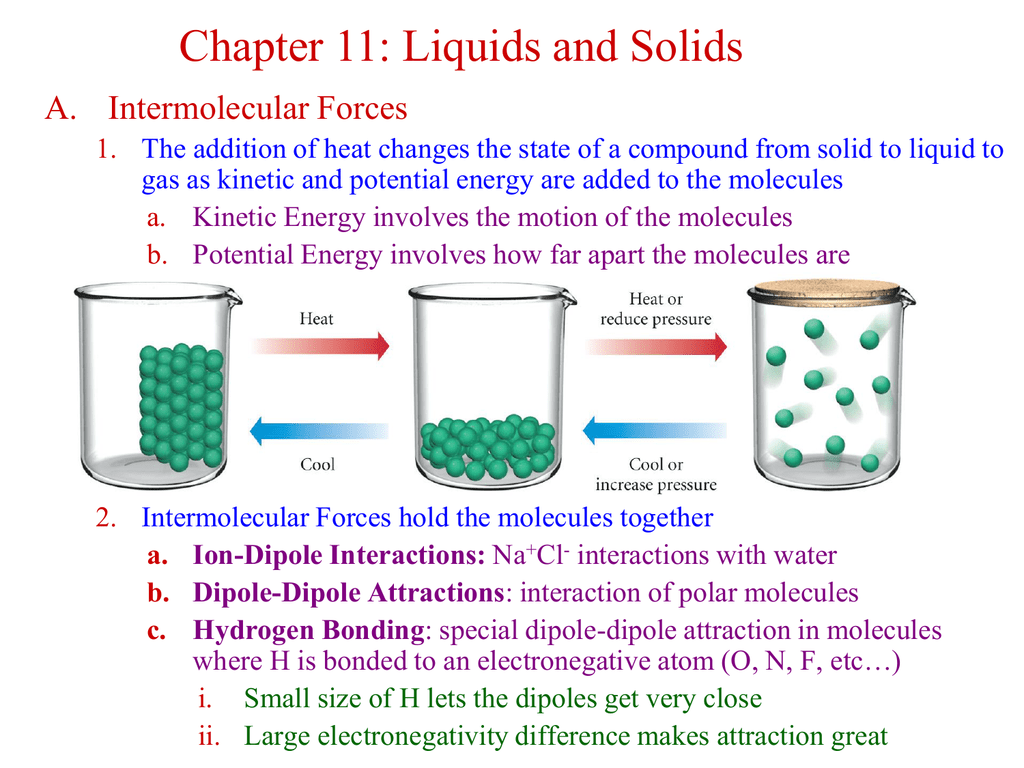
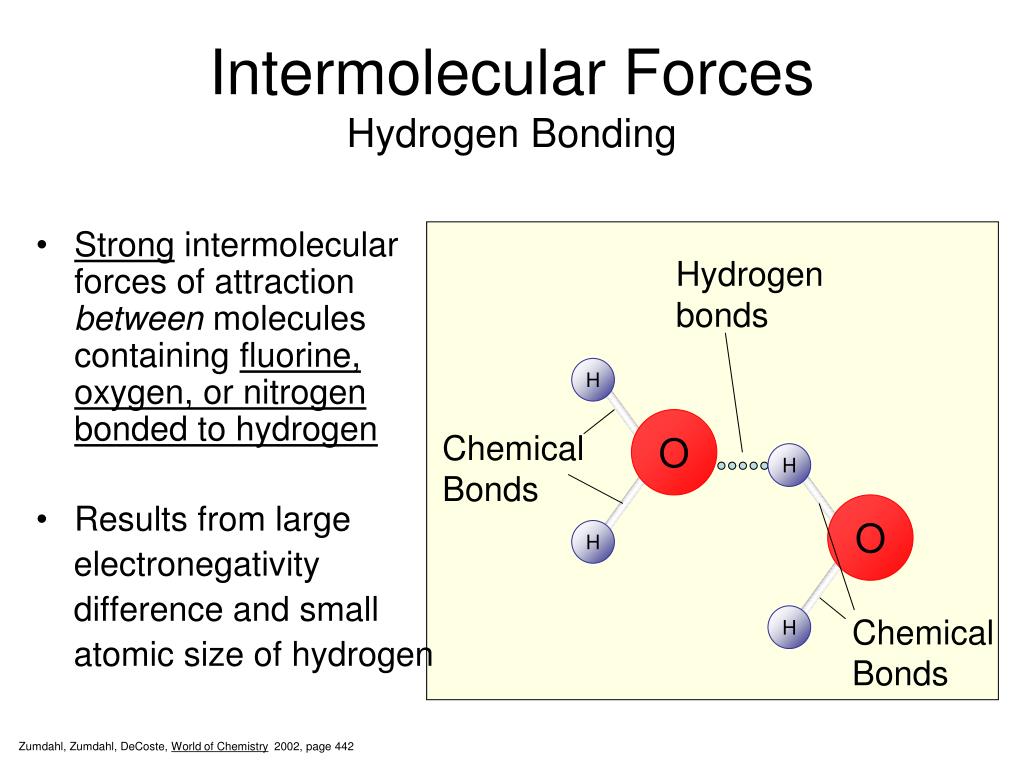
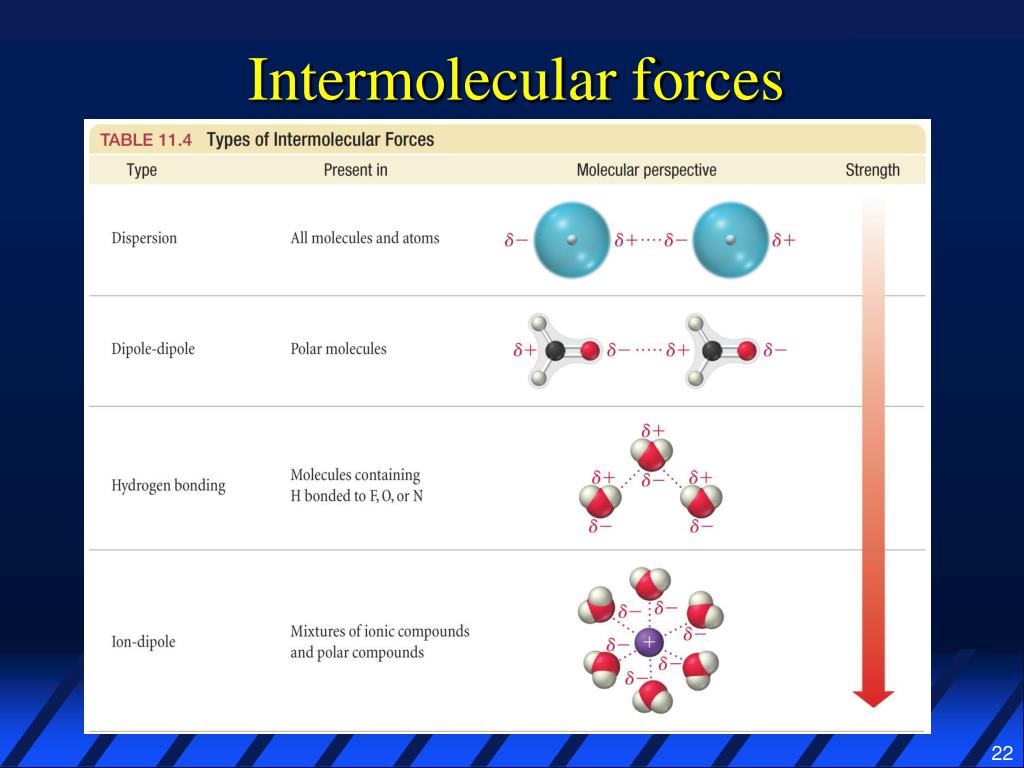
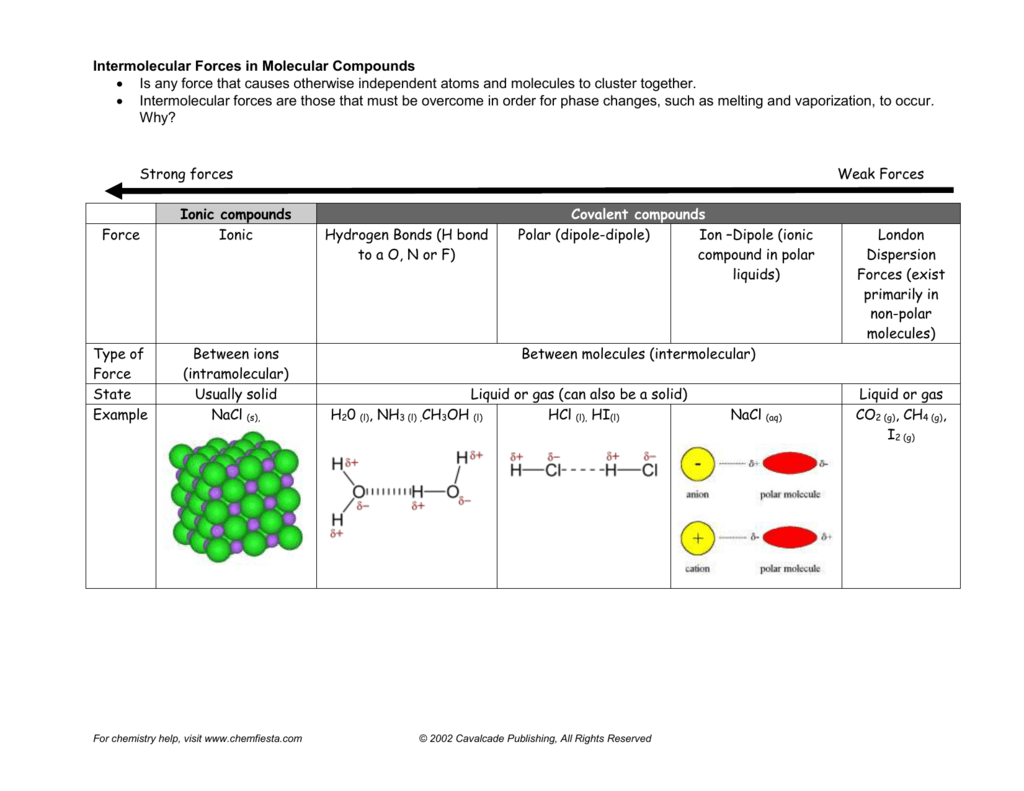
What Intermolecular Forces Are Present In Nh3 - London dispersion and hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds.
London dispersion and hydrogen bonds. The hydrogen bonds are many magnitudes. Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds.
The hydrogen bonds are many magnitudes. Every molecule experiences london dispersion as an intermolecular force. London dispersion and hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds.
Intermolecular Forces
Every molecule experiences london dispersion as an intermolecular force. The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. London dispersion and hydrogen bonds.
PPT Intermolecular Forces PowerPoint Presentation ID705859
The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. Every molecule experiences london dispersion as an intermolecular force. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. London dispersion and hydrogen bonds.
Intermolecular Force Types and Examples StudiousGuy
The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. London dispersion and hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The hydrogen bonds are many magnitudes.
Intermolecular Forces in Chemistry Intermolecular force, Chemistry
Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. The hydrogen bonds are many magnitudes. London dispersion and hydrogen bonds.
Ammonia Intermolecular Forces
London dispersion and hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. The hydrogen bonds are many magnitudes. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen.
HCl Intermolecular Forces — Type, Strong or Weak? Techiescientist
The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. Every molecule experiences london dispersion as an intermolecular force. London dispersion and hydrogen bonds. The hydrogen bonds are many magnitudes.
Intermolecular Forces
The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. London dispersion and hydrogen bonds. The hydrogen bonds are many magnitudes.
Ammonia Intermolecular Forces
Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. London dispersion and hydrogen bonds. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. The hydrogen bonds are many magnitudes.
Intermolecular And Surface Forces
London dispersion and hydrogen bonds. The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen. Every molecule experiences london dispersion as an intermolecular force. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds.
Intermolecular forces
Every molecule experiences london dispersion as an intermolecular force. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. London dispersion and hydrogen bonds. The hydrogen bonds are many magnitudes. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen.
London Dispersion And Hydrogen Bonds.
The hydrogen bonds are many magnitudes. The types of intermolecular forces present in ammonia, or nh3, are hydrogen bonds. Every molecule experiences london dispersion as an intermolecular force. In nh3 (ammonia), the intermolecular forces present are hydrogen bonding, which occurs between the hydrogen.