What Is A Zero Dipole Moment - In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. A dipole moment is a measure of the polarity between two atoms in a molecule. Explain how dipole moments depend on both molecular shape and bond polarity.
Explain how dipole moments depend on both molecular shape and bond polarity. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. A dipole moment is a measure of the polarity between two atoms in a molecule.
Explain how dipole moments depend on both molecular shape and bond polarity. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. A dipole moment is a measure of the polarity between two atoms in a molecule.
Why Is The Dipole Moment Of Benzene Zero Quora
In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. A dipole moment is a measure of the polarity between two atoms in a molecule. Explain how dipole moments depend on both molecular shape and bond polarity.
Biochemistry 1.9 Molecular Dipole Moments Draw It to Know It
A dipole moment is a measure of the polarity between two atoms in a molecule. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. Explain how dipole moments depend on both molecular shape and bond polarity.
9. What is dipole? What is zero and non zero dipole moment?
In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. Explain how dipole moments depend on both molecular shape and bond polarity. A dipole moment is a measure of the polarity between two atoms in a molecule.
Dipole Moment Periodic Table
A dipole moment is a measure of the polarity between two atoms in a molecule. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. Explain how dipole moments depend on both molecular shape and bond polarity.
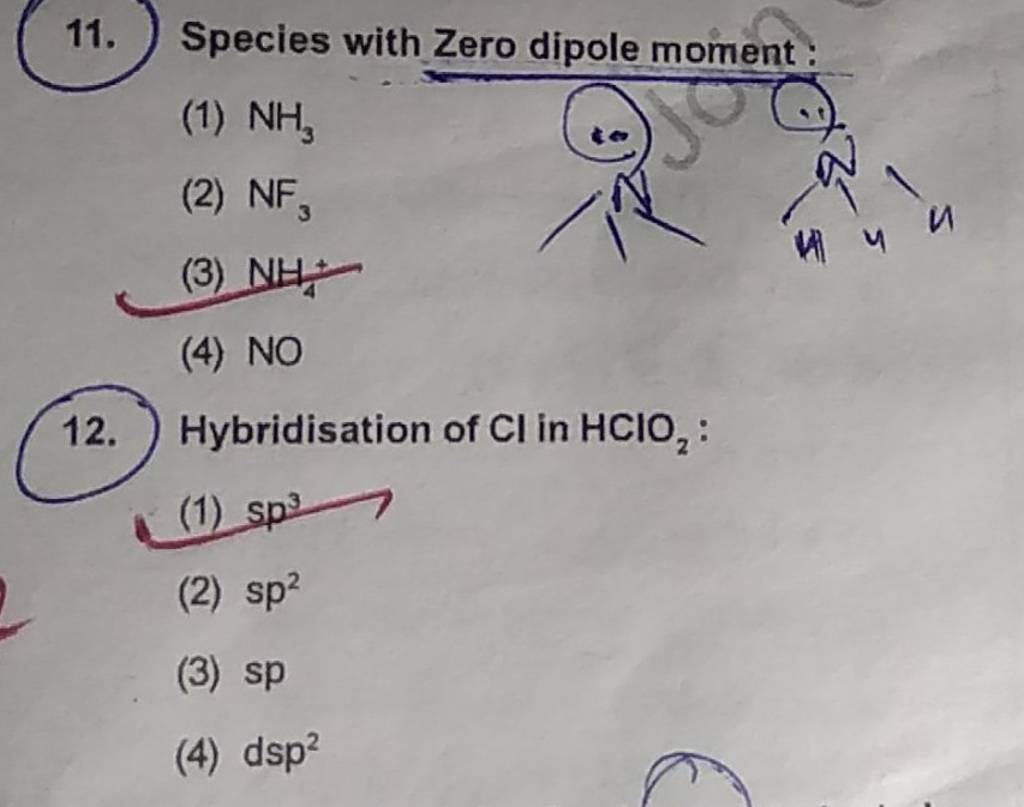
Which molecule has zero dipole moment?
Explain how dipole moments depend on both molecular shape and bond polarity. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. A dipole moment is a measure of the polarity between two atoms in a molecule.
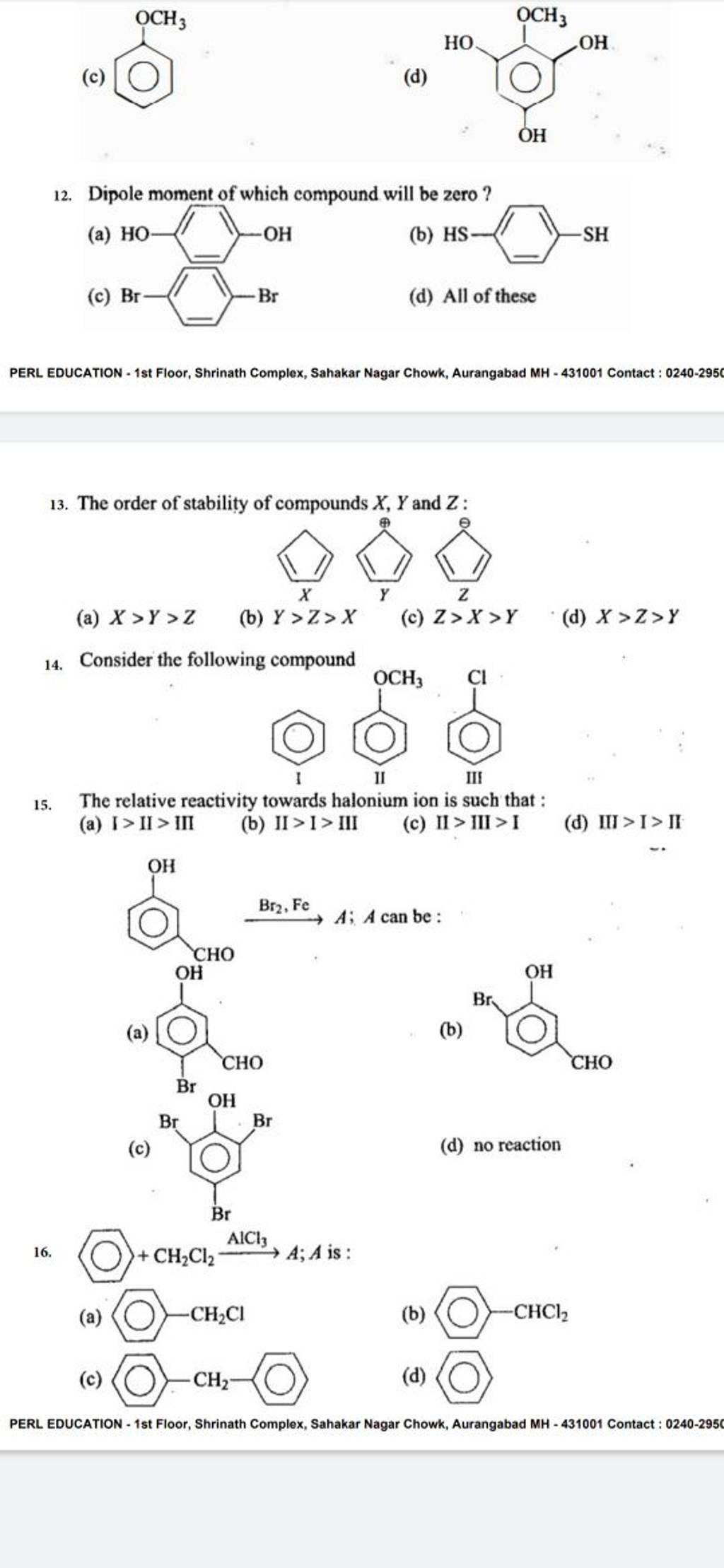
Dipole moment of which compound will be zero ? Filo
In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. Explain how dipole moments depend on both molecular shape and bond polarity. A dipole moment is a measure of the polarity between two atoms in a molecule.
Species with Zero dipole moment Filo
In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. Explain how dipole moments depend on both molecular shape and bond polarity. A dipole moment is a measure of the polarity between two atoms in a molecule.
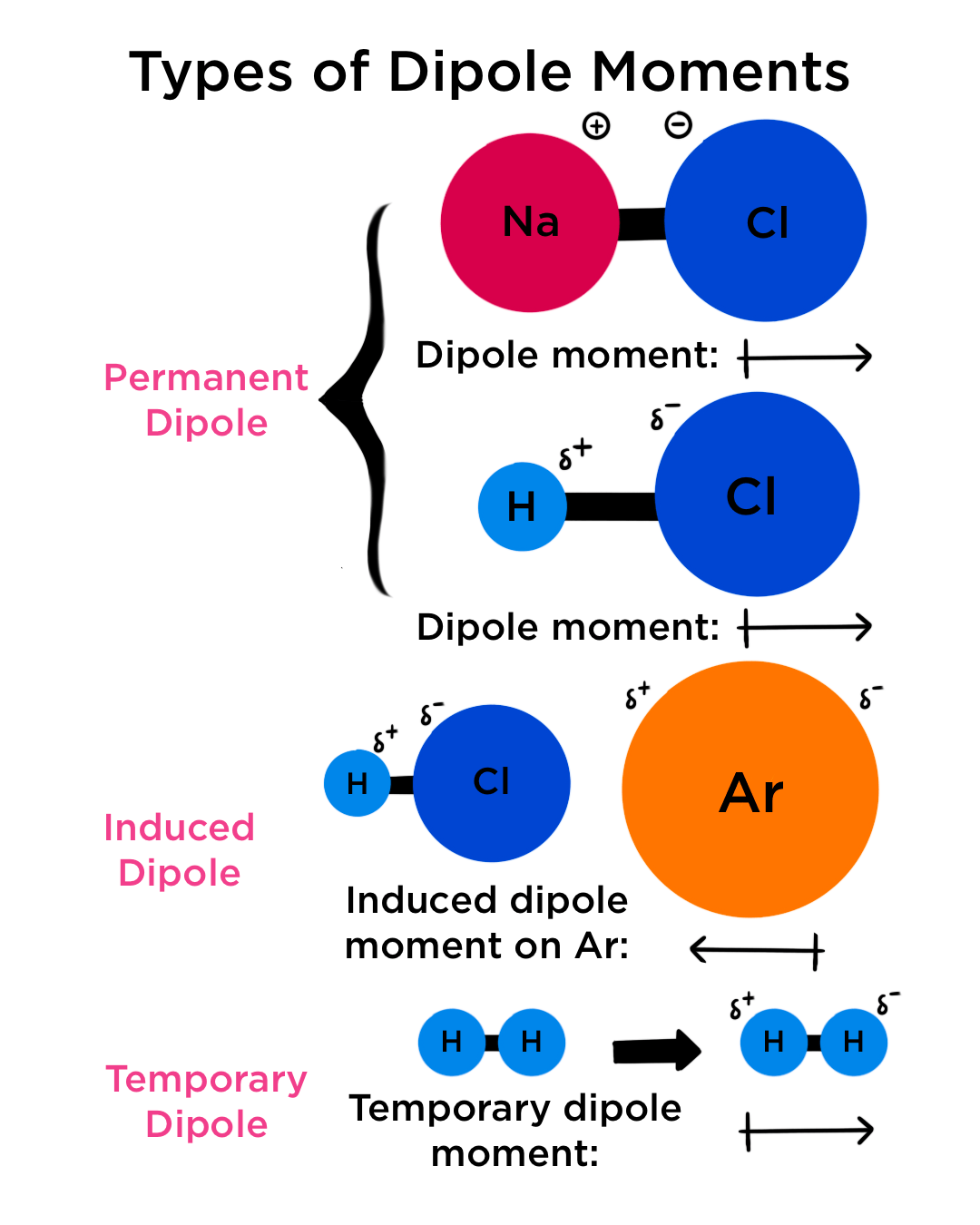
an electric moment and polarization diagram with the symbol for which
A dipole moment is a measure of the polarity between two atoms in a molecule. Explain how dipole moments depend on both molecular shape and bond polarity. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero.
Dipole moment of CH4 is zero but it's not the same for SF4 Please
Explain how dipole moments depend on both molecular shape and bond polarity. In the triatomic co 2 (carbon dioxide) molecule, the dipole moment is zero. A dipole moment is a measure of the polarity between two atoms in a molecule.
In The Triatomic Co 2 (Carbon Dioxide) Molecule, The Dipole Moment Is Zero.
A dipole moment is a measure of the polarity between two atoms in a molecule. Explain how dipole moments depend on both molecular shape and bond polarity.