What Is Always Conserved In A Chemical Reaction - This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). We can predict the masses of products and reactants involved in chemical reactions as. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Mass is conserved in chemical reactions. Why is mass always conserved in a chemical reaction? During a chemical reaction no atoms are created or destroyed. This means that there are.
This means that there are. We can predict the masses of products and reactants involved in chemical reactions as. Why is mass always conserved in a chemical reaction? During a chemical reaction no atoms are created or destroyed. Mass is conserved in chemical reactions. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants.
During a chemical reaction no atoms are created or destroyed. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Mass is conserved in chemical reactions. This means that there are. Why is mass always conserved in a chemical reaction? This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). We can predict the masses of products and reactants involved in chemical reactions as.
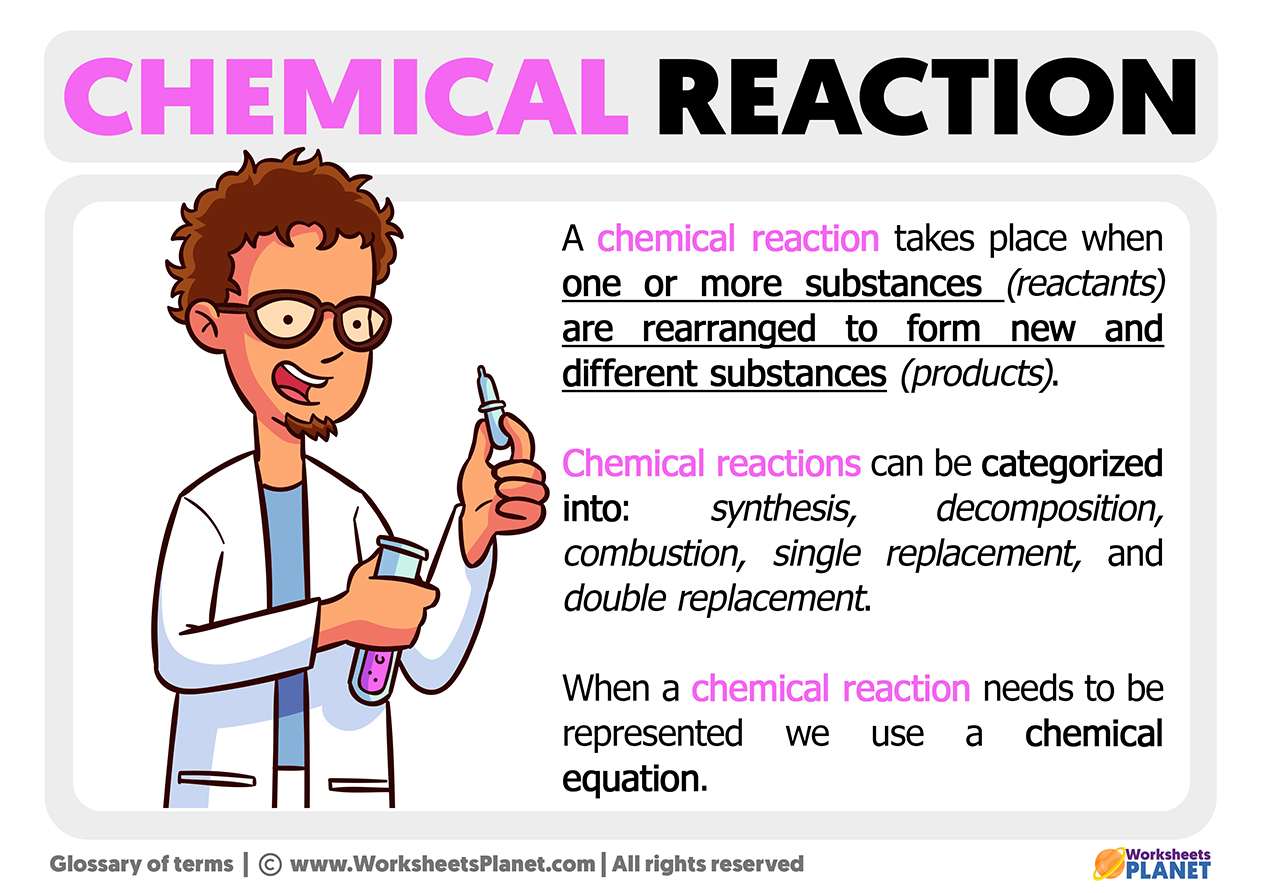
What Is A Chemical Reaction
Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). During a chemical reaction no atoms are created or destroyed. Why is mass always conserved in a chemical reaction? In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Mass is conserved.
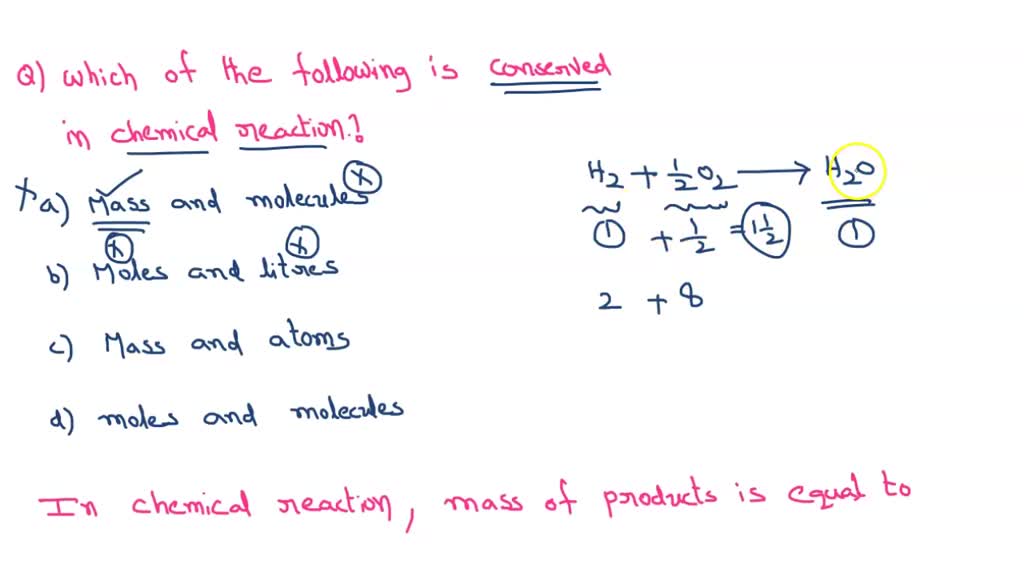
SOLVED Which of the following are CONSERVED in every chemical reaction
This means that there are. This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. Why is mass always conserved in a chemical reaction? During a chemical reaction no atoms are created or destroyed. Chemical reaction when chemical bonds are broken and made between atoms, so that.
What Is Conserved in Chemical Reactions? Sciencing
During a chemical reaction no atoms are created or destroyed. We can predict the masses of products and reactants involved in chemical reactions as. Mass is conserved in chemical reactions. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). In every chemical reaction, the same mass of matter must end up.
Chemical Reaction Definition, Types and Examples Class 10 Science
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. Why is mass always conserved in a chemical reaction? During a chemical reaction no atoms are created.
Which of these is/are conserved during a chemical reaction? Filo
Why is mass always conserved in a chemical reaction? This means that there are. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. During a chemical reaction no.
[Solved] Is mass or number of moles always conserved in a chemical
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. We can predict the masses of products and reactants involved in chemical reactions as. This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. Why is mass.
Energy Is Conserved Always Home Page
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of.
Solved 1. Which of the following is/are conserved in a
Why is mass always conserved in a chemical reaction? During a chemical reaction no atoms are created or destroyed. This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. This means that there are. Chemical reaction when chemical bonds are broken and made between atoms, so that.
Solved Which of the following is not always conserved in a
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of.
Trendy Chemical Reaction 35887296 Vector Art at Vecteezy
This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those. Mass is conserved in chemical reactions. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). This means that there are. During a chemical reaction no atoms are created.
This Means That There Are.
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements). Why is mass always conserved in a chemical reaction? This means that the mass of substances present at the start of a reaction (reactants) must be equal to the mass of those.
We Can Predict The Masses Of Products And Reactants Involved In Chemical Reactions As.
Mass is conserved in chemical reactions. During a chemical reaction no atoms are created or destroyed.