What Is Covalent Character - If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; Ionic compounds generally show partial covalent character. For example, the ionic compound licl 2 sh ows. Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. We discussed what is covalent character significance in ionic bonds. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is.
For example, the ionic compound licl 2 sh ows. Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. Ionic compounds generally show partial covalent character. If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; We discussed what is covalent character significance in ionic bonds. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is.
For example, the ionic compound licl 2 sh ows. Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. We discussed what is covalent character significance in ionic bonds. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is. Ionic compounds generally show partial covalent character. If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions;
Ionic vs Covalent Which is which and how to tell them apart
Ionic compounds generally show partial covalent character. For example, the ionic compound licl 2 sh ows. We discussed what is covalent character significance in ionic bonds. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is. If the.
How to tell if Ionic Bond or Covalent Bond Worksheets Library
For example, the ionic compound licl 2 sh ows. If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is..
Covalent Bond Definition, Examples, Types, Properties, & FAQs
Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out.
Covalent Bonding Questions and Revision MME Worksheets Library
Ionic compounds generally show partial covalent character. If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. For.
Covalent and Ionic Character (ALevel) ChemistryStudent
We discussed what is covalent character significance in ionic bonds. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is. For example, the ionic compound licl 2 sh ows. Covalent character occurs in ionic bonds when the postive.
Covalent and Ionic Character (ALevel) ChemistryStudent
If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; We discussed what is covalent character significance in ionic bonds. Ionic compounds generally show partial covalent character. For example, the ionic compound licl 2 sh ows. Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge.
Covalent Bond Biology Simple
Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. We discussed what is covalent character significance in ionic bonds. The ionic bond can have a covalent character whenever the cation draws the electron cloud of the.
Covalent bond Definition and Examples Biology Online Dictionary
If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. Ionic compounds generally show partial covalent character. The.
Covalent Bonding (Biology) — Definition & Role Expii
Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. We discussed what is covalent character significance in ionic bonds. Ionic compounds generally show partial covalent character. The ionic bond can have a covalent character whenever the.
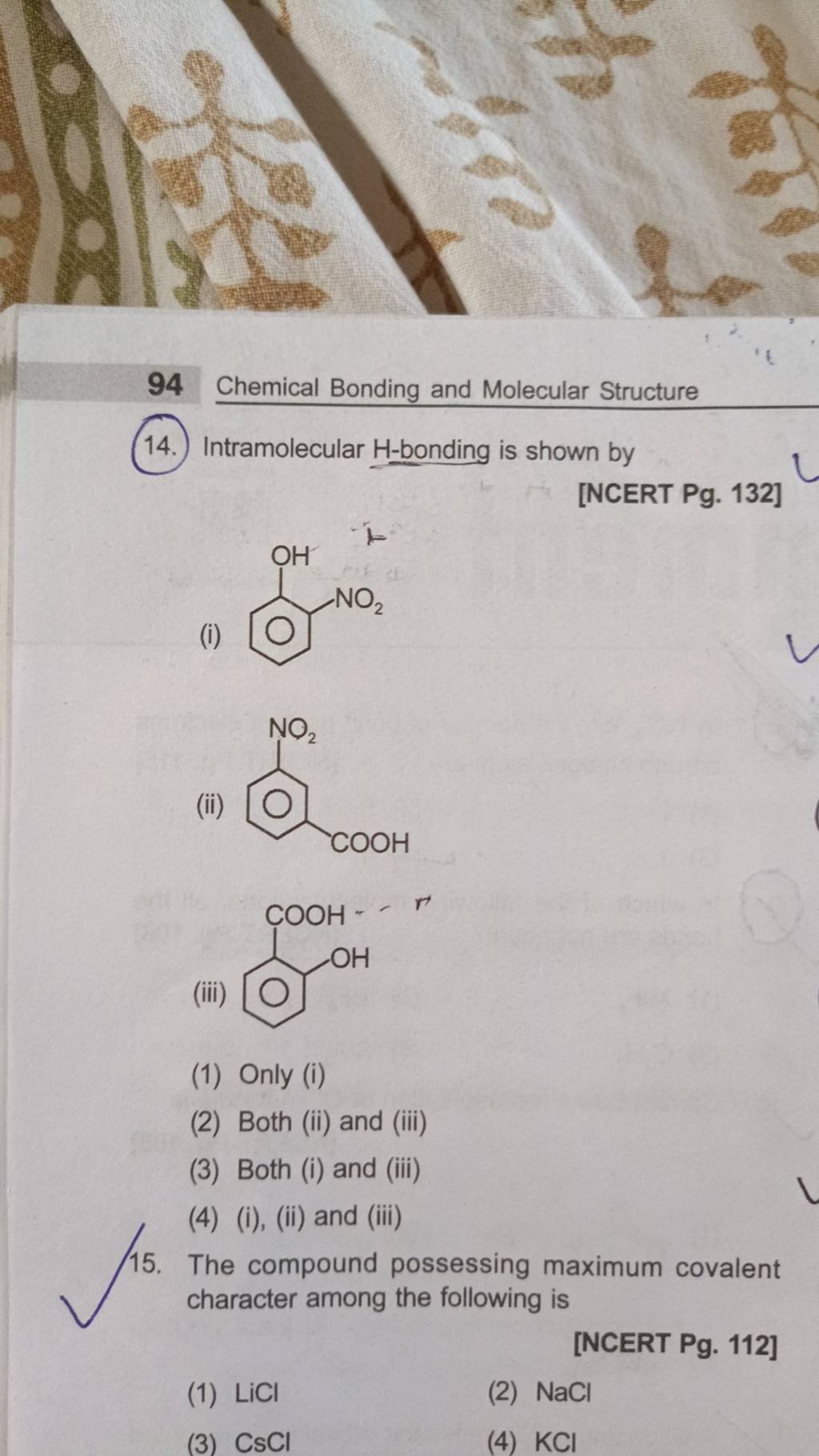
The compound possessing maximum covalent character among the following is..
Ionic compounds generally show partial covalent character. If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; Covalent character occurs in ionic bonds when the postive (usually metal) ion is highly charge dense and can polarise the counter ion causing electrons to be shared between the two ions rather than. We.
Ionic Compounds Generally Show Partial Covalent Character.
The ionic bond can have a covalent character whenever the cation draws the electron cloud of the anion, causing polarisation and the electron clouds to expand out towards the cation that is. If the electrons are pulled away from the negatively charged ion enough, the electrons are held ‘between’ the ions; We discussed what is covalent character significance in ionic bonds. For example, the ionic compound licl 2 sh ows.