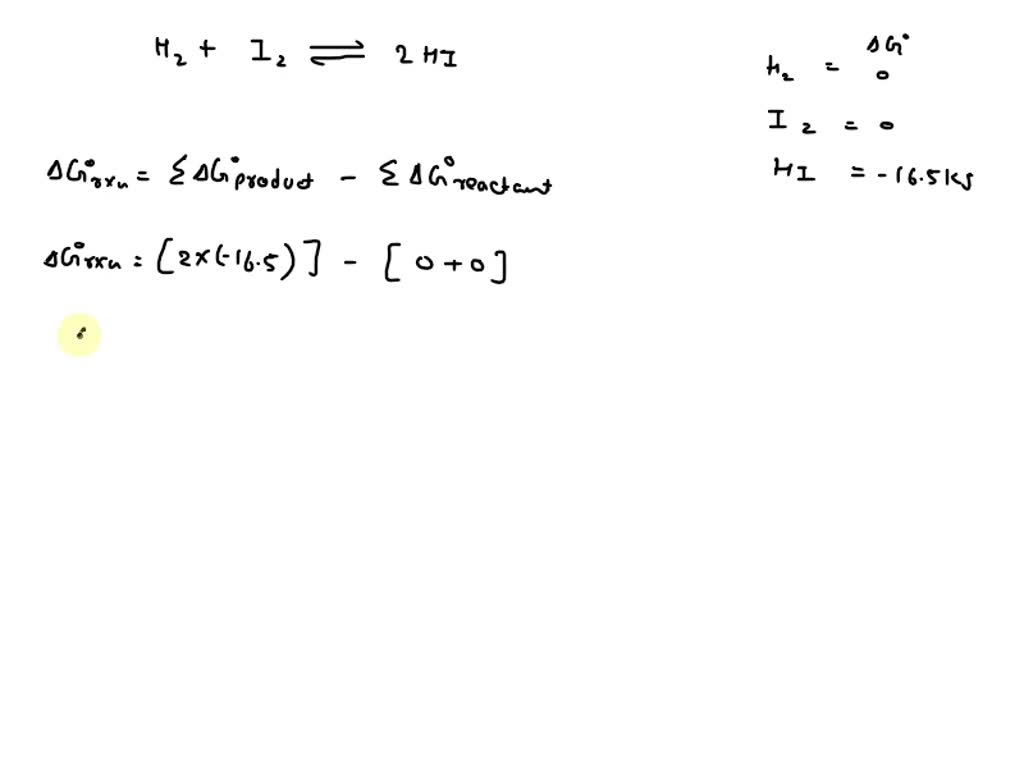What Is Kw At 25 Degrees Celsius - Kw is also known as ionic product of water and its. Bond breaking occurs when water. To calculate the kw, we determine the concentration of hydrogen and. At 25 degrees celsius, a pkw of water is 14 pkw. Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. Furthermore, why does the temperature increase kw? This fundamental chemical constant is not merely a. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴.
Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. To calculate the kw, we determine the concentration of hydrogen and. Kw is also known as ionic product of water and its. This fundamental chemical constant is not merely a. Furthermore, why does the temperature increase kw? Bond breaking occurs when water. At 25 degrees celsius, a pkw of water is 14 pkw.
At 25 degrees celsius, a pkw of water is 14 pkw. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. Furthermore, why does the temperature increase kw? Bond breaking occurs when water. Kw is also known as ionic product of water and its. This fundamental chemical constant is not merely a. To calculate the kw, we determine the concentration of hydrogen and.
25 Degrees Celsius
Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. Kw is also known as ionic product of water and its. This fundamental chemical constant is not merely a. Bond breaking occurs when.
[Solved] When the outside air temperature is 25 degrees Celsius, a
The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. At 25 degrees celsius, a pkw of water is 14 pkw. To calculate the kw, we determine the concentration of hydrogen and. Bond breaking occurs when water. Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium.
Pool thermometer showing 25 degrees Celsius Stock Photo 77889827 Alamy
This fundamental chemical constant is not merely a. Kw is also known as ionic product of water and its. Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. At 25 degrees celsius, a pkw of water is 14 pkw. Bond breaking occurs when water.
Answered At 25 degrees Celsius at 1.0018… bartleby
Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. At 25 degrees celsius, a pkw of water is 14 pkw. To calculate the kw, we determine the concentration of hydrogen and. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. This.
25 Degrees Celsius
Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. Bond breaking occurs when water. This fundamental chemical constant is not merely a. Kw is also known as ionic product of water and its. Furthermore, why does the temperature increase kw?
SOLVED Calculate ΔG* and Kp for the following equilibrium at 25
The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. At 25 degrees celsius, a pkw of water is 14 pkw. This fundamental chemical constant is not merely a. To calculate the kw, we determine the concentration of hydrogen and. Kw is also known as ionic product of water and its.
[Solved] If the pressure of a gas at 25 degrees celsius in a 250.0 L
To calculate the kw, we determine the concentration of hydrogen and. Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. At 25 degrees celsius, a pkw of water is 14 pkw. Kw.
25 Degrees Celsius Over 60 RoyaltyFree Licensable Stock Vectors
Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. Furthermore, why does the temperature increase kw? At 25 degrees celsius, a pkw of water is 14 pkw. To calculate the kw, we determine the concentration of hydrogen and. The value of kw, also known as the ion product of water,.
[Solved] If the pressure of a gas at 25 degrees celsius in a 250.0 L
Kw is also known as ionic product of water and its. This fundamental chemical constant is not merely a. Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. To calculate the kw,.
SOLVED 1. Calculate the Ksp^a+ at 25 degrees Celsius. 2. Calculate the
Bond breaking occurs when water. The value of kw, also known as the ion product of water, at 25°c is 1.0 x 10⁻¹⁴. To calculate the kw, we determine the concentration of hydrogen and. Furthermore, why does the temperature increase kw? Kw at 25 °c is the autoionization constant for water, signifying the product of hydronium and hydroxide ions' concentrations.
Kw At 25 °C Is The Autoionization Constant For Water, Signifying The Product Of Hydronium And Hydroxide Ions' Concentrations.
Kw is also known as ionic product of water and its. This fundamental chemical constant is not merely a. Furthermore, why does the temperature increase kw? To calculate the kw, we determine the concentration of hydrogen and.
The Value Of Kw, Also Known As The Ion Product Of Water, At 25°C Is 1.0 X 10⁻¹⁴.
At 25 degrees celsius, a pkw of water is 14 pkw. Bond breaking occurs when water.