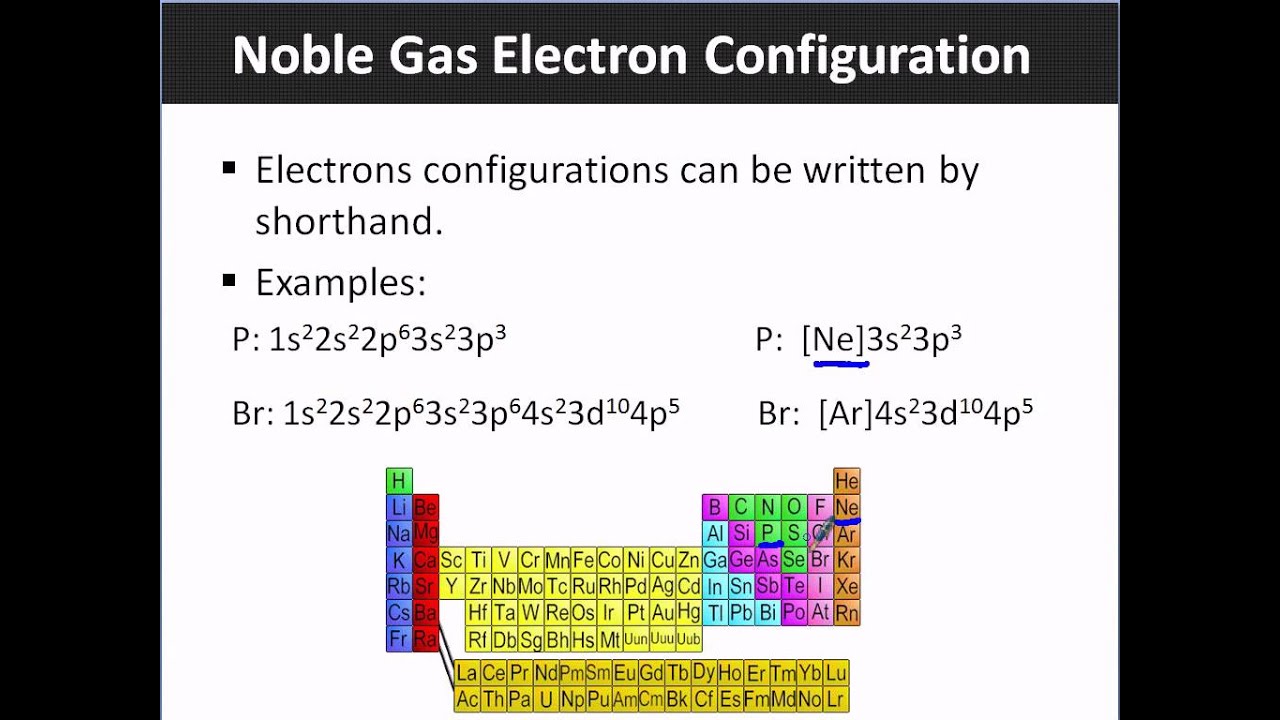
What Is Pseudo Noble Gas Configuration - It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It is achieved when an atom. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration.
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,.
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. It is achieved when an atom. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a.
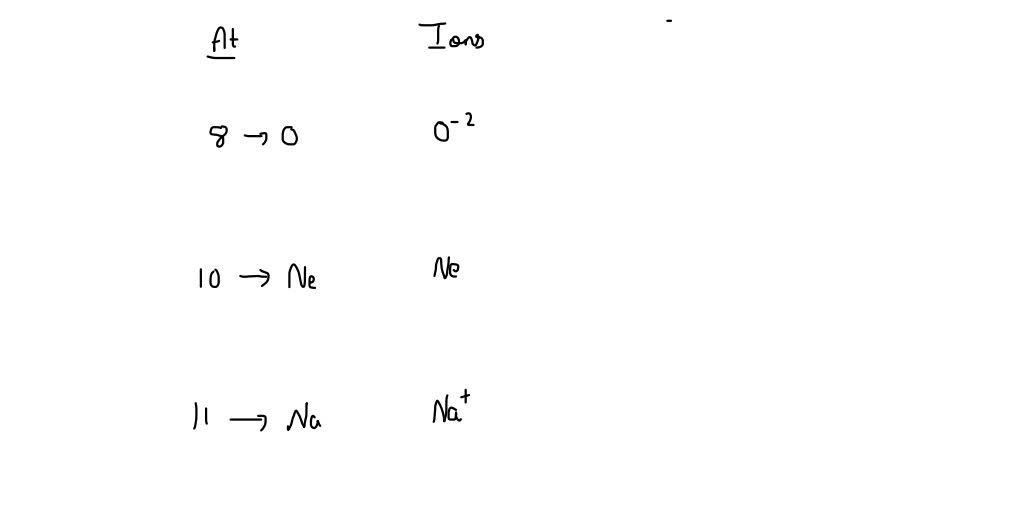
SOLVED Give the Pseudo Noble gas configuration if 8,10,11 ions
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It is achieved when an atom. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas.
Noble Gas Configuration Of Vanadium
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It is achieved when an atom. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom.
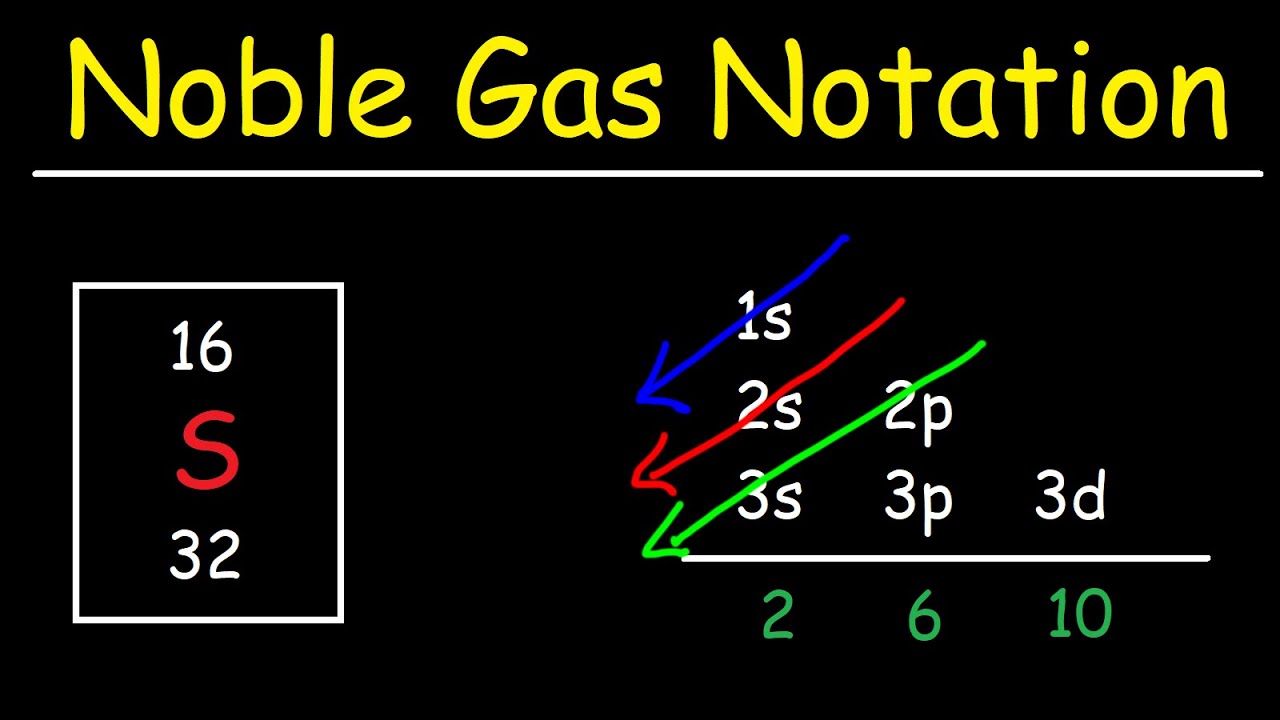
44+ noble gas electron configuration calculator EileenSuzie
It is achieved when an atom. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom.
Antimony noble gas configuration virtmilitary
Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the.
SECTIONB 86. Pseudo inert gas configuration is Filo
Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an.
SOLVEDWhat is a pseudonoble gas configuration? Give an example of one
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It is achieved when an atom. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas.
How to Write a Noble Gas Configuration for Atoms of an Element
Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p.
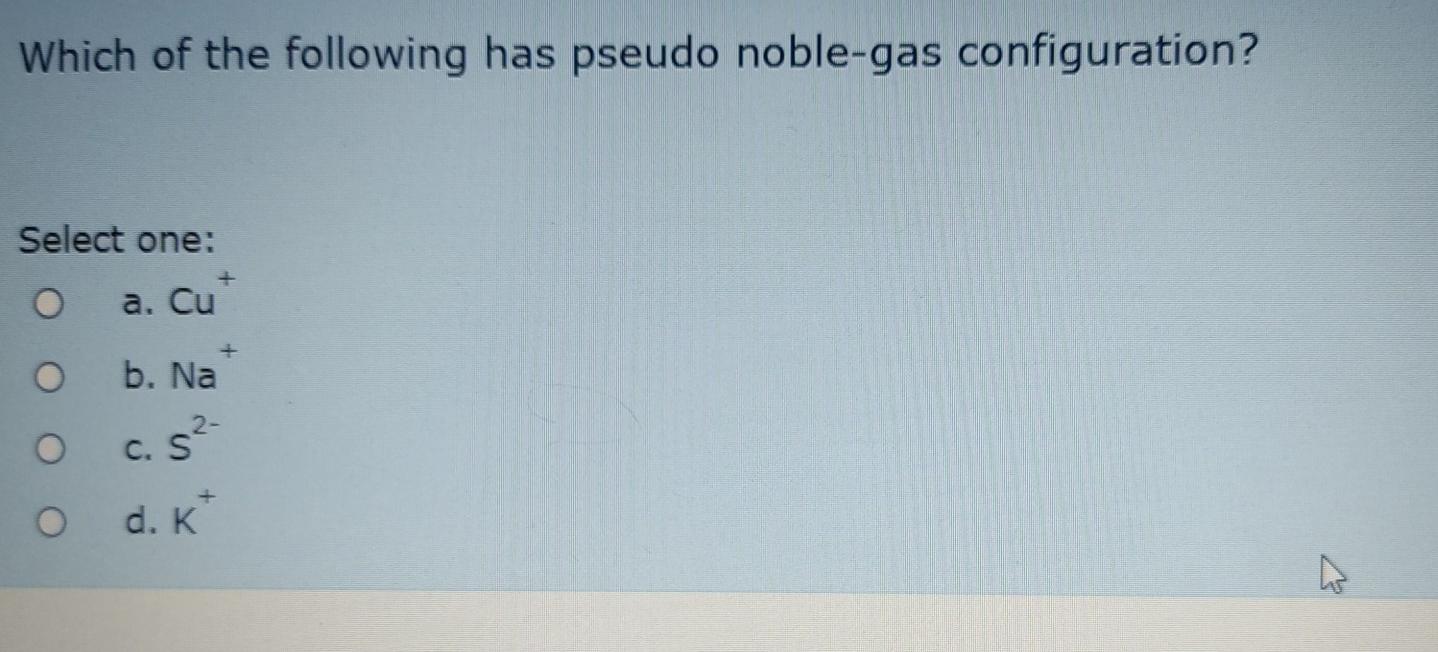
Solved Which of the following has pseudo noblegas
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron.
Noble Gas Configuration Practice Questions
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It is achieved when an atom. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo.
Noble Gas Configuration For Cobalt Ash in The Wild
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two.
Yes, The Electron Structure Of A Zinc Ion (Zn2+) Achieves A Pseudo Noble Gas Configuration By Losing Two Electrons To Have A.
It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons.