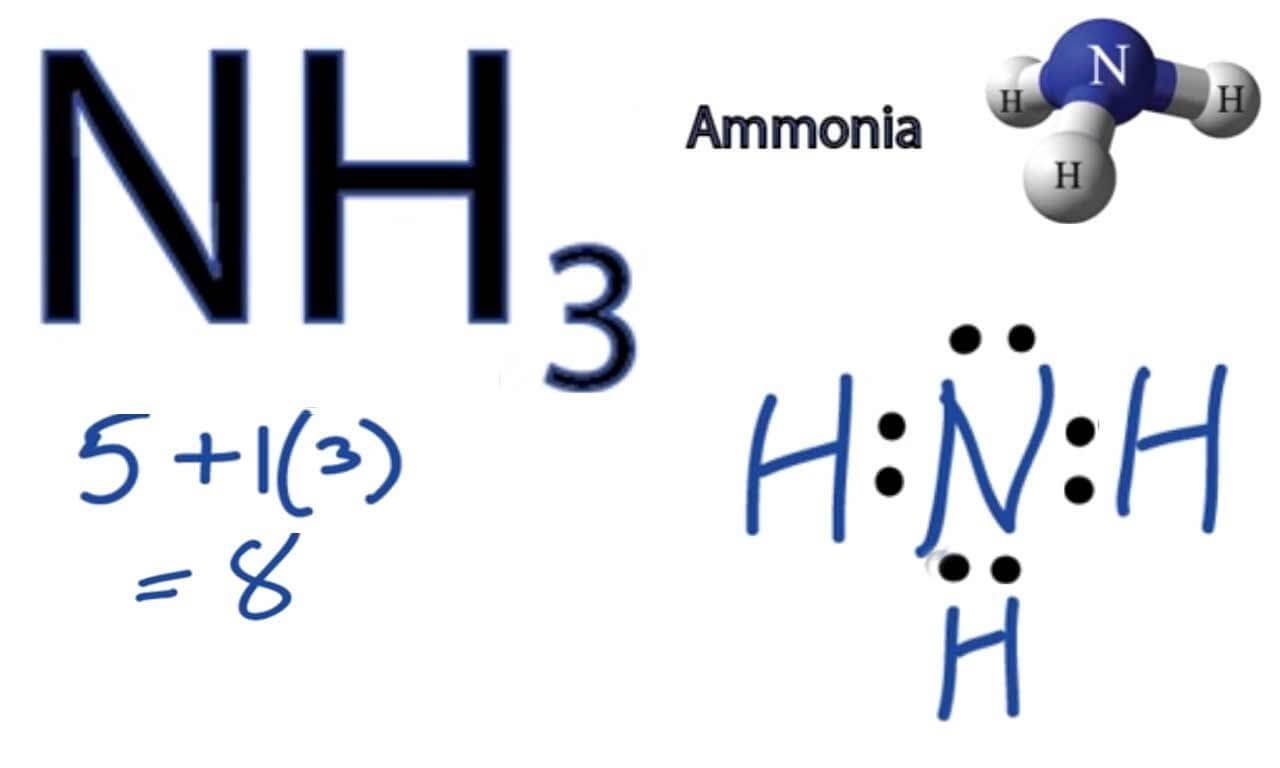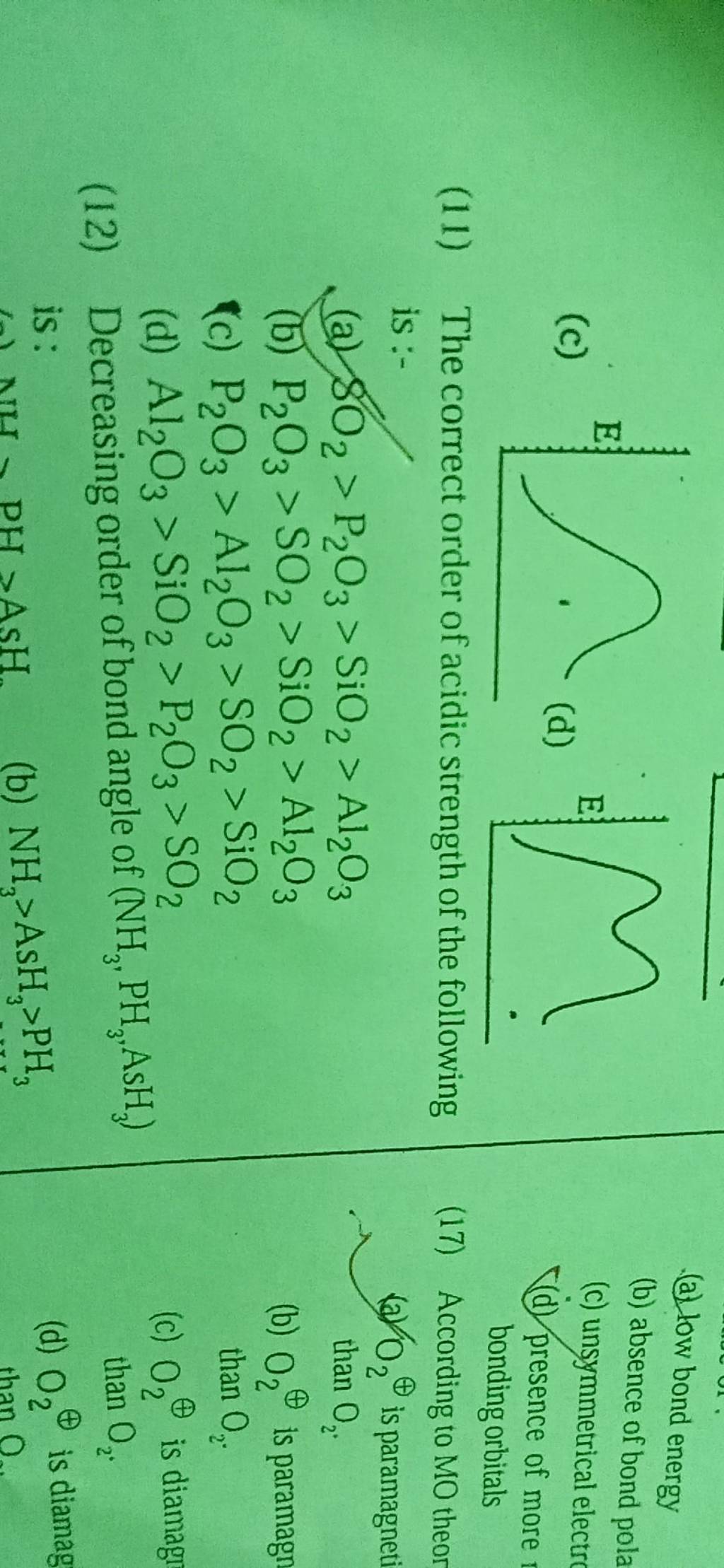What Is The Bond Angle Of Nh3 - The bond angle of nh3 is approximately 107 degrees. However, in ammonia, the bond angle is slightly less than. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The ideal bond angle for a tetrahedral arrangement is 109.5 0. This is due to the presence of the lone pair on the nitrogen atom,. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to.
The ideal bond angle for a tetrahedral arrangement is 109.5 0. However, in ammonia, the bond angle is slightly less than. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. The bond angle of nh3 is approximately 107 degrees. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. This is due to the presence of the lone pair on the nitrogen atom,.
The ideal bond angle for a tetrahedral arrangement is 109.5 0. The bond angle of nh3 is approximately 107 degrees. However, in ammonia, the bond angle is slightly less than. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. This is due to the presence of the lone pair on the nitrogen atom,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
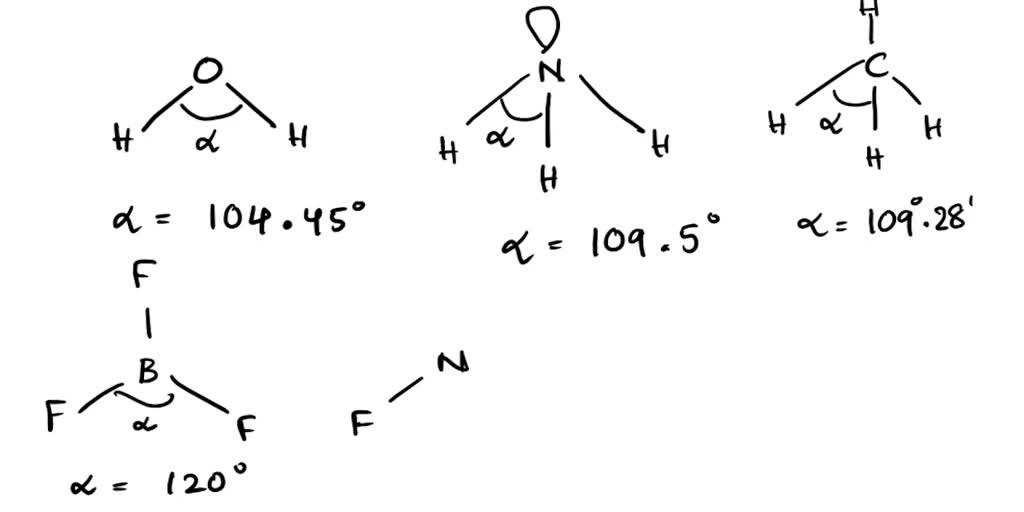
Correct order of bond angle in the given molecules (1) H2 O>NH3 >CH4
However, in ammonia, the bond angle is slightly less than. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The bond angle of nh3 is approximately 107 degrees. This.
NH3 Molecular Geometry, Hybridization, Bond Angle and Molecular Shape
This is due to the presence of the lone pair on the nitrogen atom,. The ideal bond angle for a tetrahedral arrangement is 109.5 0. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The bond angle of nh3 is approximately 107 degrees. However, in ammonia, the bond angle.
Why nh3 has more bond angle than nf3 Chemistry Chemical Bonding and
The bond angle of nh3 is approximately 107 degrees. However, in ammonia, the bond angle is slightly less than. The ideal bond angle for a tetrahedral arrangement is 109.5 0. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. Although the bond angle should be 109.5 degrees for trigonal.
Trigonal Pyramidal Bond Angle
The ideal bond angle for a tetrahedral arrangement is 109.5 0. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. This is due to the presence of the lone pair on the nitrogen atom,. However, in ammonia, the bond angle is slightly less than. Although the bond angle should.
Explain why the bond angle of NH3 is greater than that of NF3 while the
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. However, in ammonia, the bond angle is slightly less than. The ideal bond angle for a tetrahedral arrangement is 109.5.
SOLVED Bond angle in nh3 is greater than bond angle in a s h 3
The bond angle of nh3 is approximately 107 degrees. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. However, in ammonia, the bond angle is slightly less than. The ideal bond angle for a tetrahedral arrangement is 109.5 0. This is due to the presence of the lone pair.
SOLVED Compare the bond angle predicted from VSEPR Theory and the bond
The ideal bond angle for a tetrahedral arrangement is 109.5 0. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. However, in ammonia, the bond angle is slightly less than. The bond angle of nh3 is approximately 107 degrees. Nh_3 has a bond angle of about 106.67^@, while ph_3.
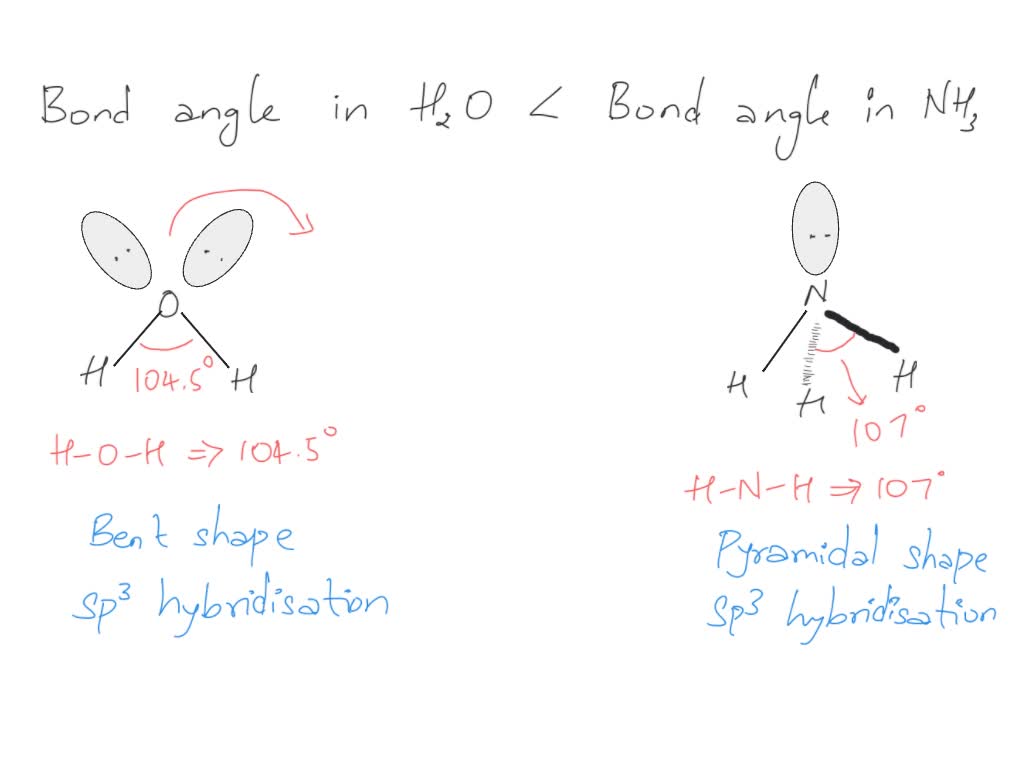
ii) Bond angle of NH3 is than H2O. Justify
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. This is due to the presence of the lone pair on the nitrogen atom,. The ideal bond angle for a tetrahedral arrangement is 109.5 0. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases.
Explain why the bond angle of NH3 is greater than that of NF3 while the
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. However, in ammonia, the bond angle is slightly less than. The bond angle of nh3 is approximately 107 degrees. The ideal bond angle for a tetrahedral arrangement is 109.5 0. Although the bond angle should be 109.5 degrees for trigonal.
Decreasing order of bond angle of (NH3 ,PH3 ,AsH3 ) Filo
Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. This is due to the presence of the lone pair on the nitrogen atom,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The bond angle of nh3 is.
However, In Ammonia, The Bond Angle Is Slightly Less Than.
Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to. The bond angle of nh3 is approximately 107 degrees. The ideal bond angle for a tetrahedral arrangement is 109.5 0. This is due to the presence of the lone pair on the nitrogen atom,.