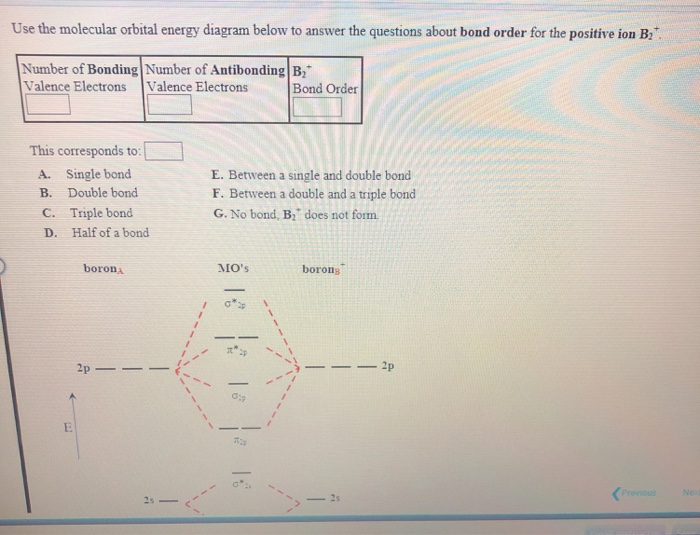
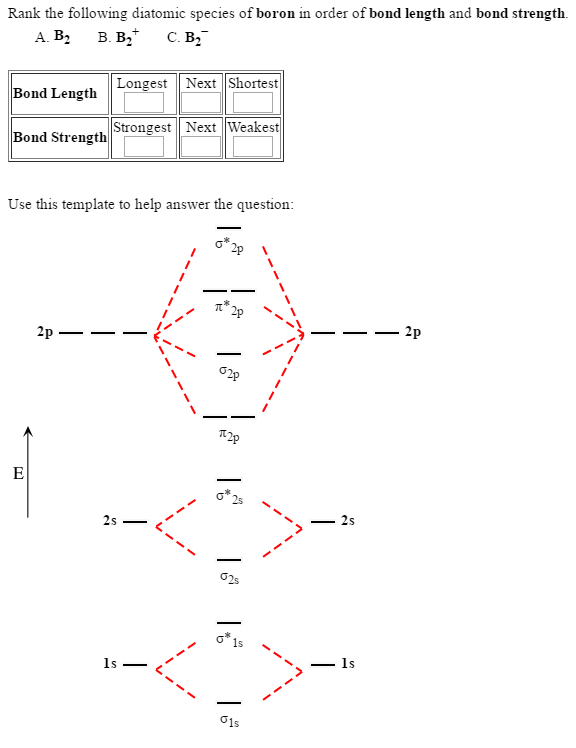
What Is The Bond Order Of B2 - Bond order in a b2 molecule is the number of chemical bonds between two boron. The bond order of a molecule can be calculated. What is bond order in a b2 molecule? The b 2 molecule is known to be formed by the combination of two boron atoms, where they. The bond order for b2+ is 1.5. The bond order of b 2 molecule: The bond order of b₂₋ is 1.5. B₂₋ has an unpaired electron and is paramagnetic. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and.
B₂₋ has an unpaired electron and is paramagnetic. The bond order of a molecule can be calculated. The bond order of b₂₋ is 1.5. The bond order for b2+ is 1.5. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and. Bond order in a b2 molecule is the number of chemical bonds between two boron. The b 2 molecule is known to be formed by the combination of two boron atoms, where they. The bond order of b 2 molecule: What is bond order in a b2 molecule?
The bond order of b₂₋ is 1.5. Bond order in a b2 molecule is the number of chemical bonds between two boron. The bond order of a molecule can be calculated. What is bond order in a b2 molecule? The bond order for b2+ is 1.5. The b 2 molecule is known to be formed by the combination of two boron atoms, where they. The bond order of b 2 molecule: B₂₋ has an unpaired electron and is paramagnetic. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and.
31+ calculate the bond order of b2 MarionCorrin
The b 2 molecule is known to be formed by the combination of two boron atoms, where they. The bond order for b2+ is 1.5. The bond order of a molecule can be calculated. What is bond order in a b2 molecule? The bond order of b₂₋ is 1.5.
Understanding B2 Molecule Molecular Orbital Diagram and Bond Order
The b 2 molecule is known to be formed by the combination of two boron atoms, where they. B₂₋ has an unpaired electron and is paramagnetic. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and. What is bond order in a b2 molecule? The bond order.
Understanding B2 Molecule Molecular Orbital Diagram and Bond Order
The b 2 molecule is known to be formed by the combination of two boron atoms, where they. What is bond order in a b2 molecule? B₂₋ has an unpaired electron and is paramagnetic. Bond order in a b2 molecule is the number of chemical bonds between two boron. The bond order of b 2 molecule:
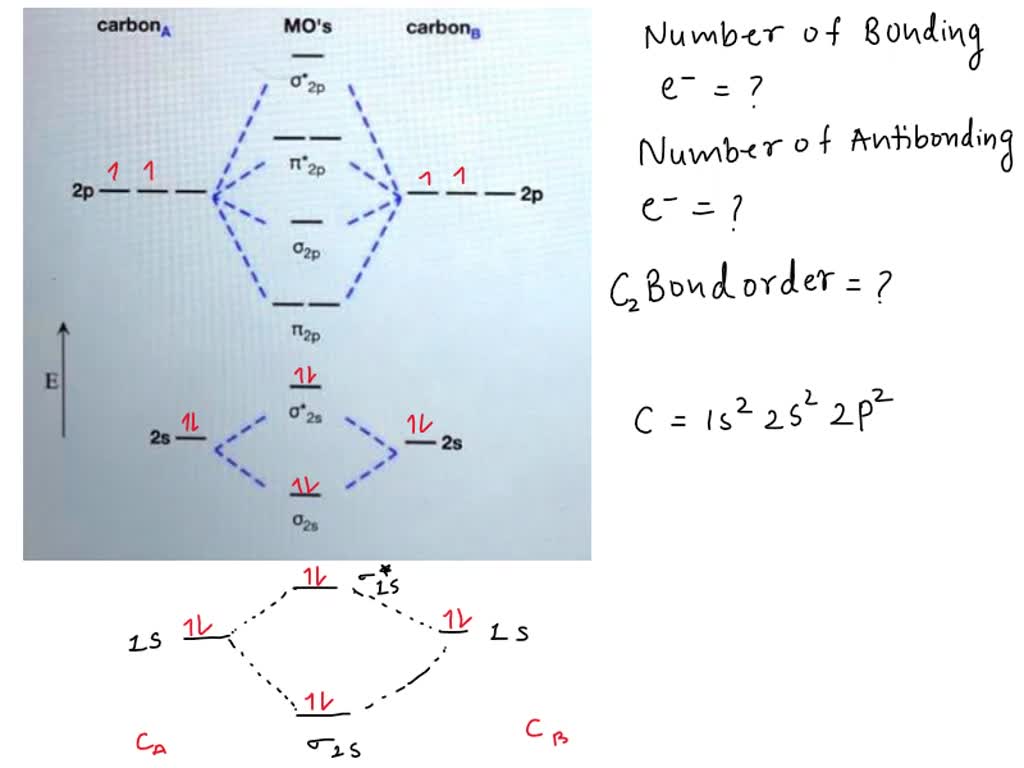
define bond order and calculate the bond order in B2 Chemistry
Bond order in a b2 molecule is the number of chemical bonds between two boron. The bond order for b2+ is 1.5. The bond order of a molecule can be calculated. B₂₋ has an unpaired electron and is paramagnetic. The bond order of b₂₋ is 1.5.
Understanding B2 Molecule Molecular Orbital Diagram and Bond Order
The bond order for b2+ is 1.5. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and. B₂₋ has an unpaired electron and is paramagnetic. What is bond order in a b2 molecule? Bond order in a b2 molecule is the number of chemical bonds between two.
Understanding B2 Molecule Molecular Orbital Diagram and Bond Order
What is bond order in a b2 molecule? The bond order of b₂₋ is 1.5. B₂₋ has an unpaired electron and is paramagnetic. The bond order of a molecule can be calculated. The b 2 molecule is known to be formed by the combination of two boron atoms, where they.
31+ calculate the bond order of b2 MarionCorrin
Bond order in a b2 molecule is the number of chemical bonds between two boron. What is bond order in a b2 molecule? The bond order for b2+ is 1.5. The bond order of a molecule can be calculated. The bond order of b 2 molecule:
SOLVED What is the bond order?
The bond order of b 2 molecule: The b 2 molecule is known to be formed by the combination of two boron atoms, where they. The bond order for b2+ is 1.5. The bond order of a molecule can be calculated. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the.
SOLVED Q1. Calculate the bond order. Q2 Calculate the bond order. Q3
The bond order of b 2 molecule: B₂₋ has an unpaired electron and is paramagnetic. What is bond order in a b2 molecule? Bond order in a b2 molecule is the number of chemical bonds between two boron. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding.
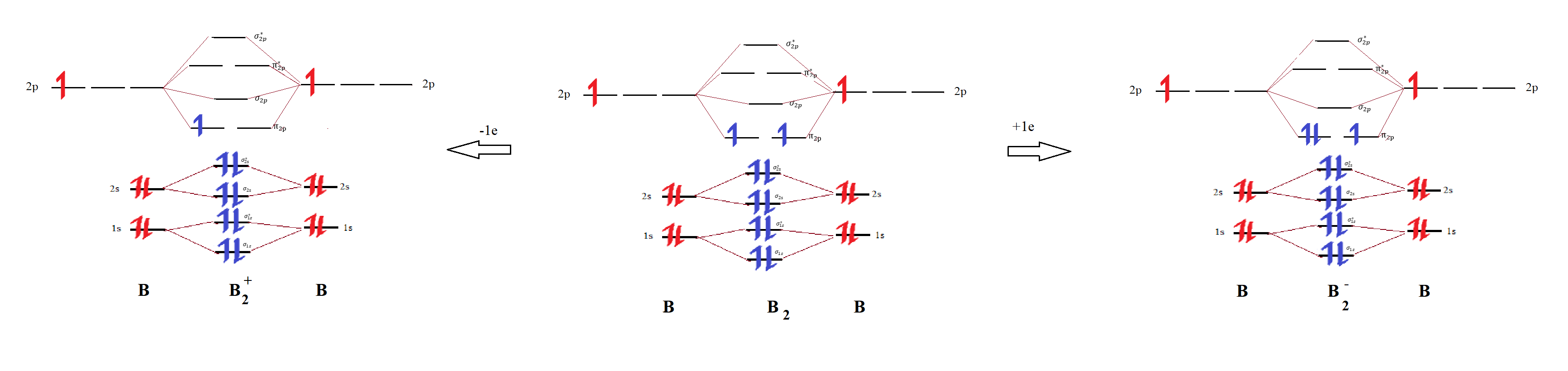
Solved Rank The Following Diatomic Species Of Boron In Or...
The b 2 molecule is known to be formed by the combination of two boron atoms, where they. The bond order of b 2 molecule: Bond order in a b2 molecule is the number of chemical bonds between two boron. What is bond order in a b2 molecule? The bond order of a molecule can be calculated.
By Analyzing The Mo Diagram For B2, We Can Determine The Bond Order, Which Is The Difference Between The Number Of Bonding And.
The bond order of b₂₋ is 1.5. The bond order for b2+ is 1.5. The bond order of a molecule can be calculated. What is bond order in a b2 molecule?
The B 2 Molecule Is Known To Be Formed By The Combination Of Two Boron Atoms, Where They.
Bond order in a b2 molecule is the number of chemical bonds between two boron. B₂₋ has an unpaired electron and is paramagnetic. The bond order of b 2 molecule: