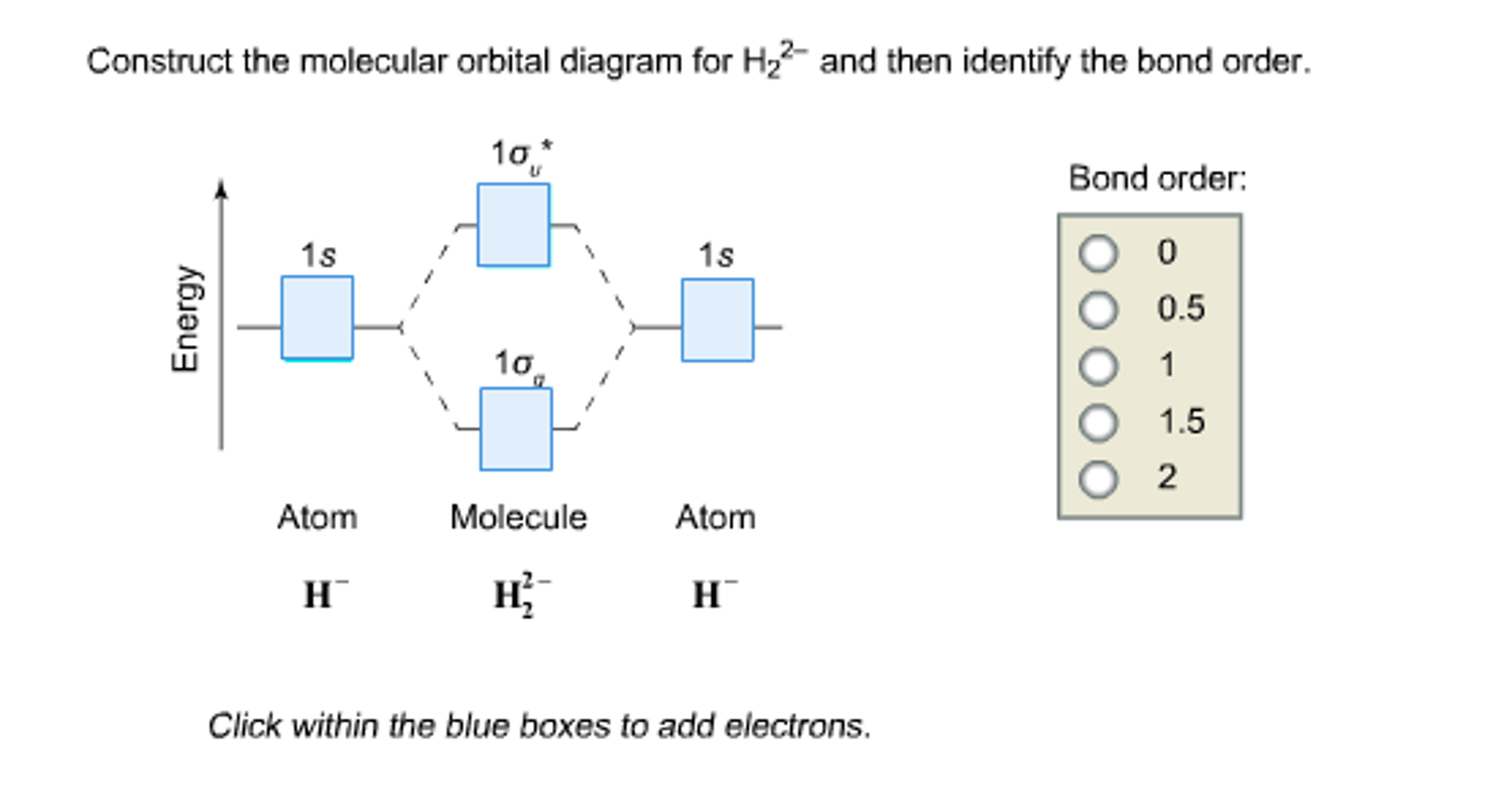
What Is The Bond Order Of H2 - The bond order of h 2− ion is 1 2. This result corresponds to the single. If it has 2 bonding electrons, calculate the number of antibonding electrons. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and.
The bond order of h 2− ion is 1 2. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. This result corresponds to the single. If it has 2 bonding electrons, calculate the number of antibonding electrons.
The bond order of h 2− ion is 1 2. If it has 2 bonding electrons, calculate the number of antibonding electrons. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. This result corresponds to the single.
Solved Construct the molecular orbital diagram for H_2^2
If it has 2 bonding electrons, calculate the number of antibonding electrons. The bond order of h 2− ion is 1 2. This result corresponds to the single. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and.
The calculated bond order in H2− ion is Filo
This result corresponds to the single. The bond order of h 2− ion is 1 2. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. If it has 2 bonding electrons, calculate the number of antibonding electrons.
Molecular Orbital Diagram For H2 And Bond Order
Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. This result corresponds to the single. If it has 2 bonding electrons, calculate the number of antibonding electrons. The bond order of h 2− ion is 1 2.
What is Bond order of H2? UO Chemists
If it has 2 bonding electrons, calculate the number of antibonding electrons. The bond order of h 2− ion is 1 2. This result corresponds to the single. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and.
38 The bond order of H2+ is Filo
If it has 2 bonding electrons, calculate the number of antibonding electrons. The bond order of h 2− ion is 1 2. This result corresponds to the single. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and.
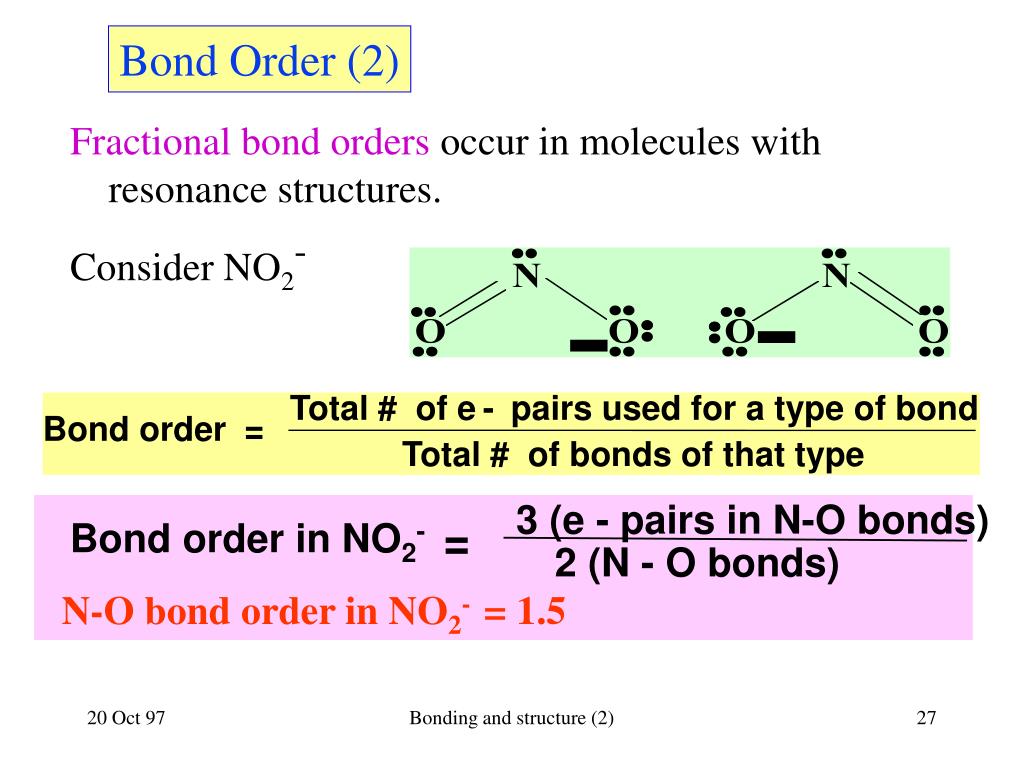
Bond Order Diagram
This result corresponds to the single. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. The bond order of h 2− ion is 1 2. If it has 2 bonding electrons, calculate the number of antibonding electrons.
Define Bond Order. Calculate The Bond Order Of C2, H2 And N2. Chemistry
The bond order of h 2− ion is 1 2. If it has 2 bonding electrons, calculate the number of antibonding electrons. This result corresponds to the single. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and.
How To Calculate Bond Order From Mo Diagram General Wiring Diagram
The bond order of h 2− ion is 1 2. This result corresponds to the single. If it has 2 bonding electrons, calculate the number of antibonding electrons. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and.
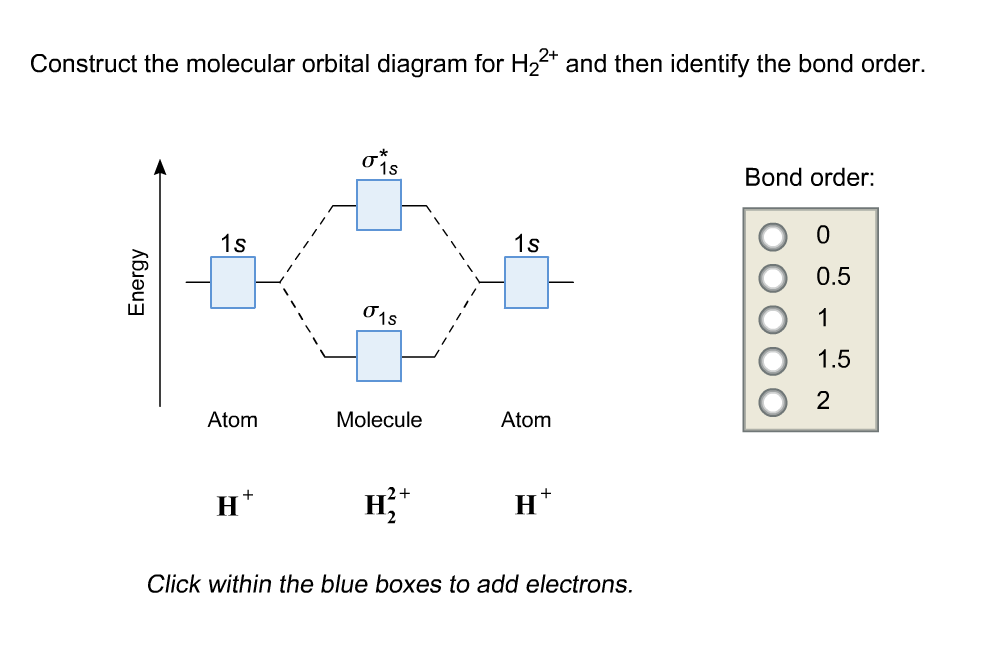
Solved Construct the molecular orbital diagram for H22+ and
Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. The bond order of h 2− ion is 1 2. If it has 2 bonding electrons, calculate the number of antibonding electrons. This result corresponds to the single.
[Solved] what is the bond order of the hydrogen to hydrogen bond after
The bond order of h 2− ion is 1 2. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. If it has 2 bonding electrons, calculate the number of antibonding electrons. This result corresponds to the single.
This Result Corresponds To The Single.
Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to mo theory to form one σ1s and. The bond order of h 2− ion is 1 2. If it has 2 bonding electrons, calculate the number of antibonding electrons.