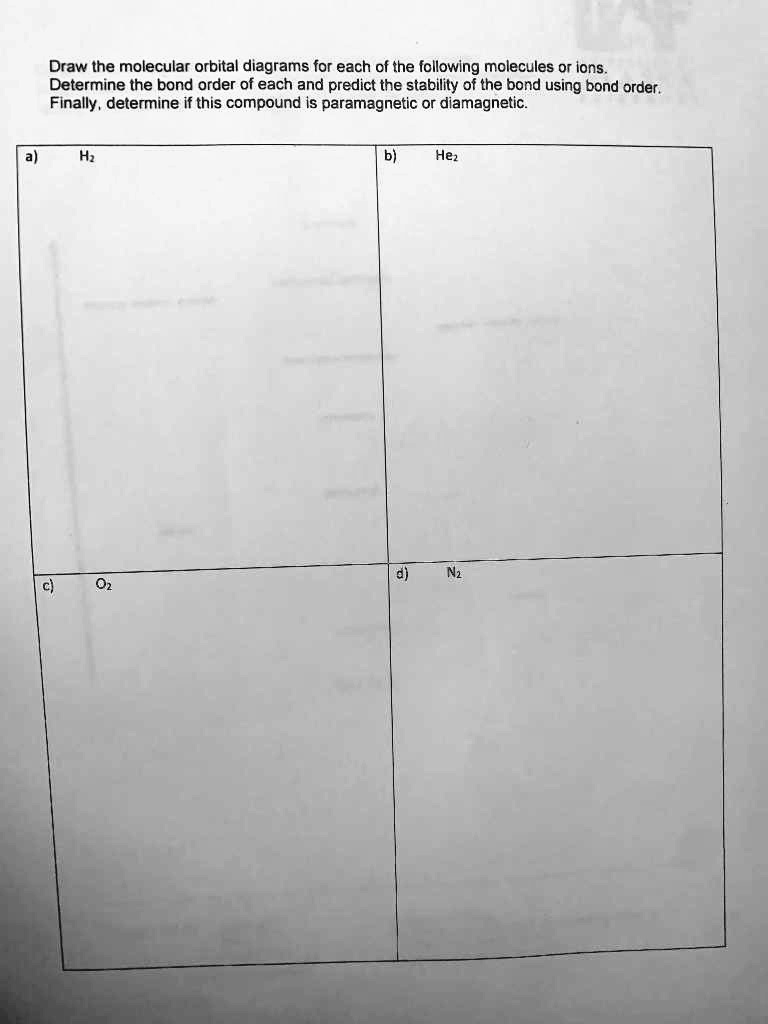
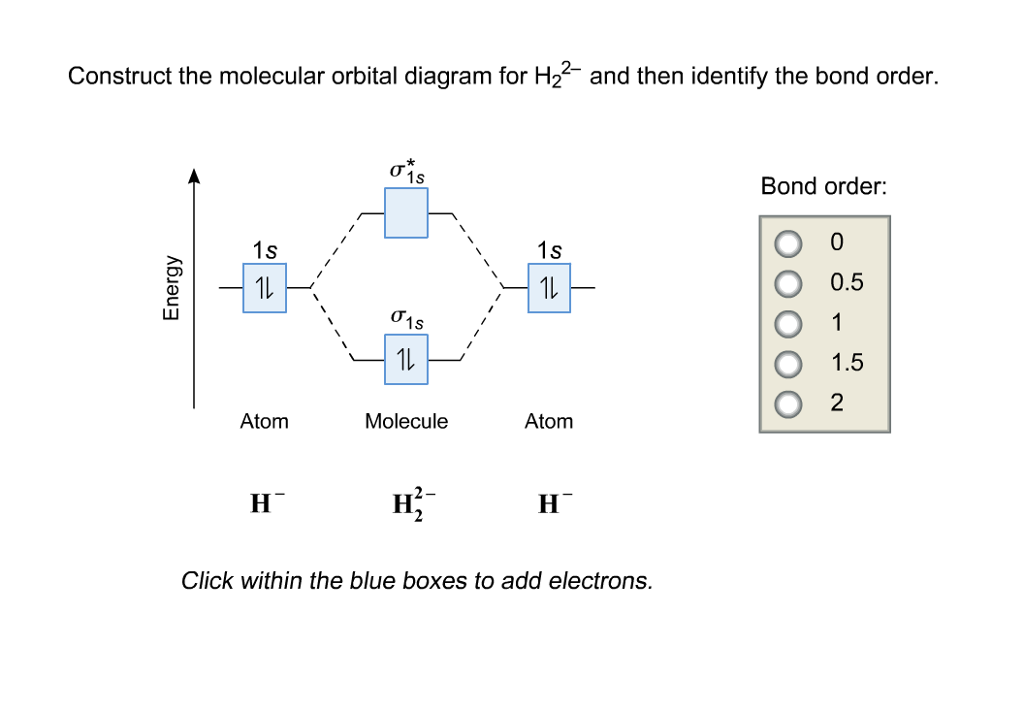
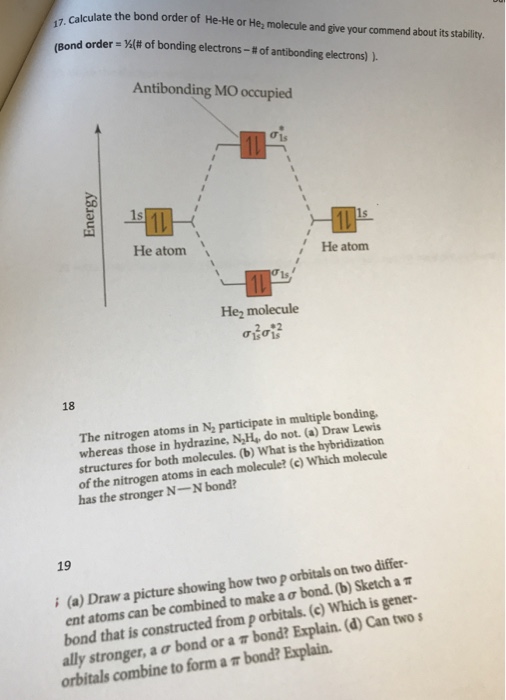
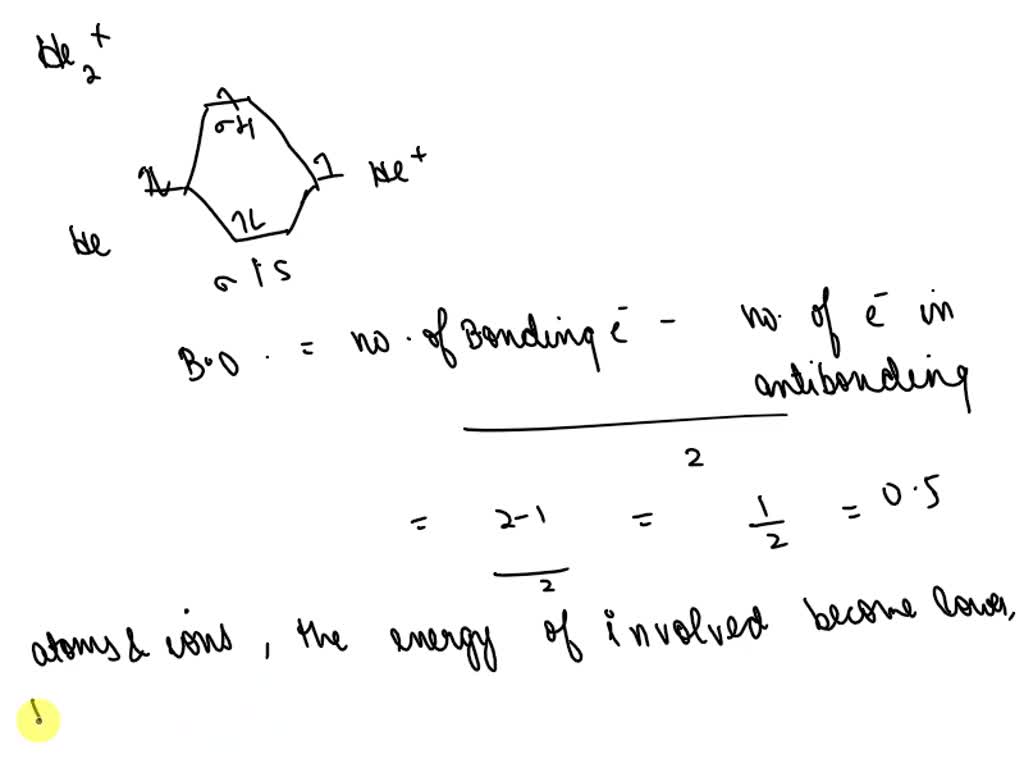What Is The Bond Order Of He2 - Bond order is 0.5, and. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. The he2+ ion is formed by removing two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. This means that the bond between the two. Here’s the best way to solve it. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. [2] this chemical is the largest diatomic.
Bond order is 0.5, and. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. [2] this chemical is the largest diatomic. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. The he2+ ion is formed by removing two. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. This means that the bond between the two. Here’s the best way to solve it.
Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. [2] this chemical is the largest diatomic. Here’s the best way to solve it. This means that the bond between the two. Bond order is 0.5, and. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. The he2+ ion is formed by removing two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing.
What is the bond order of the He2 molecule Answer as fast as you can
The he2+ ion is formed by removing two. This means that the bond between the two. [2] this chemical is the largest diatomic. Here’s the best way to solve it. Bond order is 0.5, and.
SOLVED Draw the molecular orbital diagrams for each of the following
This means that the bond between the two. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. [2] this chemical is the largest diatomic. The he2+ ion is formed by removing two. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions.
bond order of He_2^+ is
The he2+ ion is formed by removing two. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. Bond order is 0.5, and. To find the bond order.
SOLVED What is the bond order of the He2+ ion? Would you expect this
[2] this chemical is the largest diatomic. This means that the bond between the two. Bond order is 0.5, and. The he2+ ion is formed by removing two. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms.
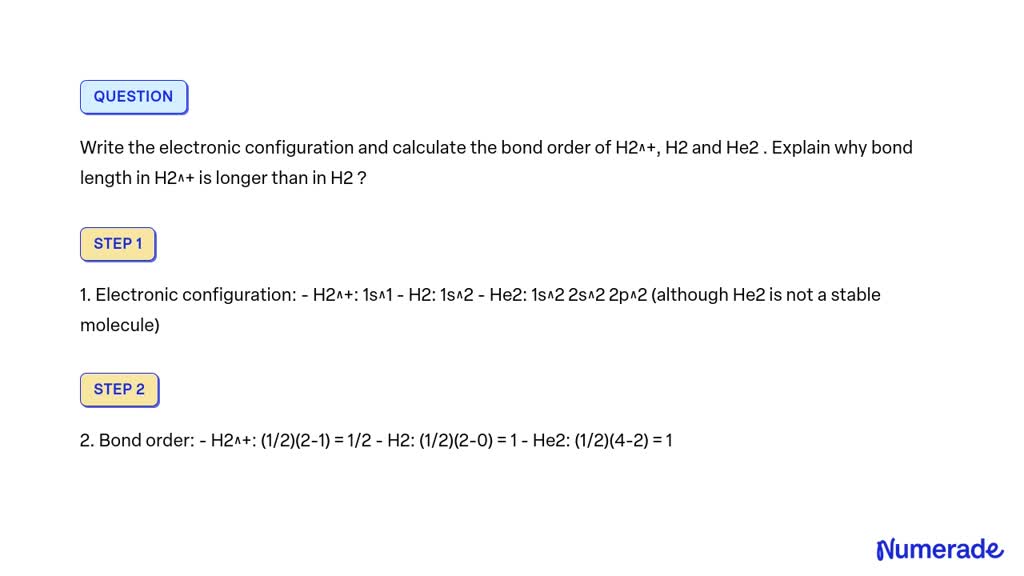
SOLVED Write the electronic configuration and calculate the bond order
The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. This means that the bond between the two. Bond order is 0.5, and. To find the bond order.
OneClass The bond order of a He2+ ion is a. 0 b. 0.5 c. 1 d. 1.5 e. 2
The he2+ ion is formed by removing two. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. This means that the bond between the two. Here’s the best way to solve it. We can determine the bond order of a molecule by subtracting the antibonding.
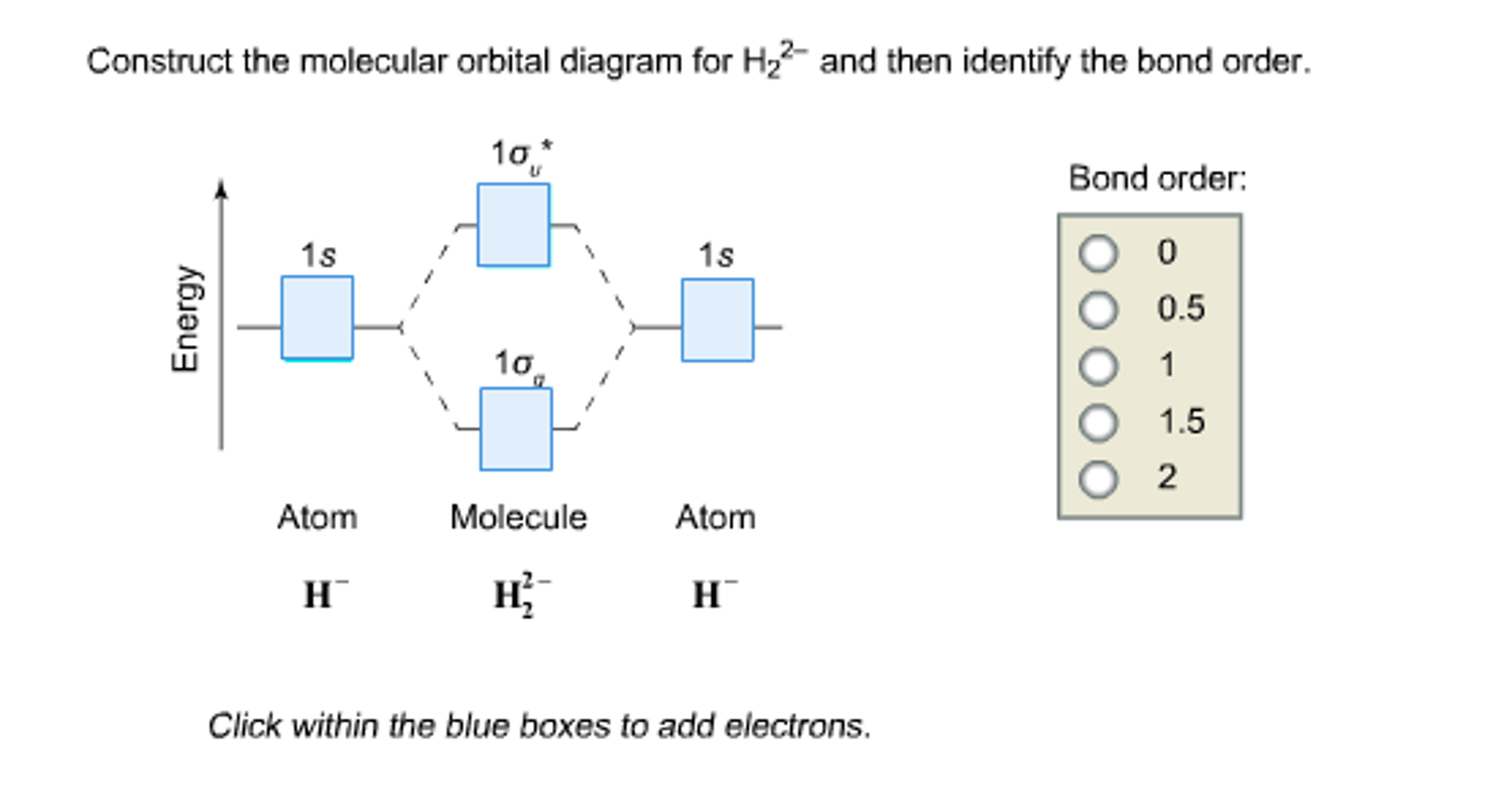
Solved Construct the molecular orbital diagram for H_2^2
To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. Bond order is 0.5, and. The helium dimer is a van der waals molecule with formula he 2 consisting of.
Solved Construct the molecular orbital diagram for H2 and
We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. [2] this chemical is the largest diatomic. Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. Here’s the best way to solve it. To find the.
What is Bond order of H2? UO Chemists
We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. Here’s the best way to solve it. The helium dimer is a van der waals molecule with formula he 2.
[2] This Chemical Is The Largest Diatomic.
Draw an mo diagram to predict the bond order and stability of an he2+ molecule formed from one he atom and one he+ ion. The bond order of the he2+ ion is 1, indicating a single bond between the two helium ions. The helium dimer is a van der waals molecule with formula he 2 consisting of two helium atoms. The he2+ ion is formed by removing two.
Bond Order Is 0.5, And.
Here’s the best way to solve it. We can determine the bond order of a molecule by subtracting the antibonding (destablizing electrons) from the bonding (stabilizing. To find the bond order of h e 2 +, first determine the number of bonding electrons and antibonding electrons by. This means that the bond between the two.