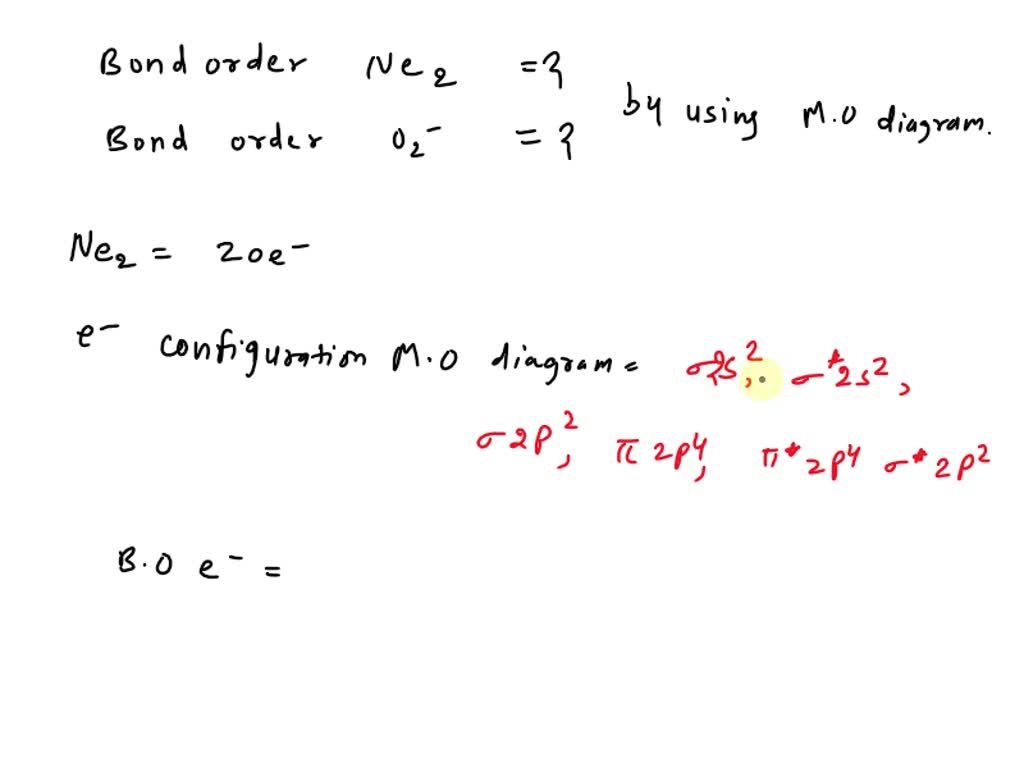What Is The Bond Order Of Ne2 - This process involves the sharing. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The molecular orbital diagram of ne2+ has 8 valence. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. The molecular orbital diagram shows the bonding and antibonding. The bond order of ne2 is 0. The bond order of ne2 is 0. This means that ne2 does not form a stable bond and does not exist as a molecule. According to the molecular orbital theory, the bond order of ne2 can be determined. The bond order of ne2+ according to molecular orbital theory is 1.5.
This process involves the sharing. The molecular orbital diagram of ne2+ has 8 valence. This means that ne2 does not form a stable bond and does not exist as a molecule. The bond order of ne2 is 0. According to the molecular orbital theory, the bond order of ne2 can be determined. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The bond order of ne2+ according to molecular orbital theory is 1.5. The molecular orbital diagram shows the bonding and antibonding. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. The bond order of ne2 is 0.
This process involves the sharing. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. The bond order of ne2 is 0. This means that ne2 does not form a stable bond and does not exist as a molecule. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The molecular orbital diagram of ne2+ has 8 valence. The bond order of ne2+ according to molecular orbital theory is 1.5. The molecular orbital diagram shows the bonding and antibonding. According to the molecular orbital theory, the bond order of ne2 can be determined. The bond order of ne2 is 0.
Calculate the bond order of N2 and N2 and predict its
The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The bond order of ne2+ according to molecular orbital theory is 1.5. The bond order of ne2 is 0. The bond order of ne2 is 0. According to the molecular orbital theory, the bond order of ne2 can be determined.
SOLVED Apply molecular orbital theory to determine the bond order of
This process involves the sharing. According to the molecular orbital theory, the bond order of ne2 can be determined. The molecular orbital diagram of ne2+ has 8 valence. The bond order of ne2 is 0. The bond order of ne2+ according to molecular orbital theory is 1.5.
⏩SOLVEDPlace the following molecules and ions in order from… Numerade
The bond order of ne2 is 0. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The molecular orbital diagram of ne2+ has 8 valence. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. According to the molecular orbital theory, the bond order of.
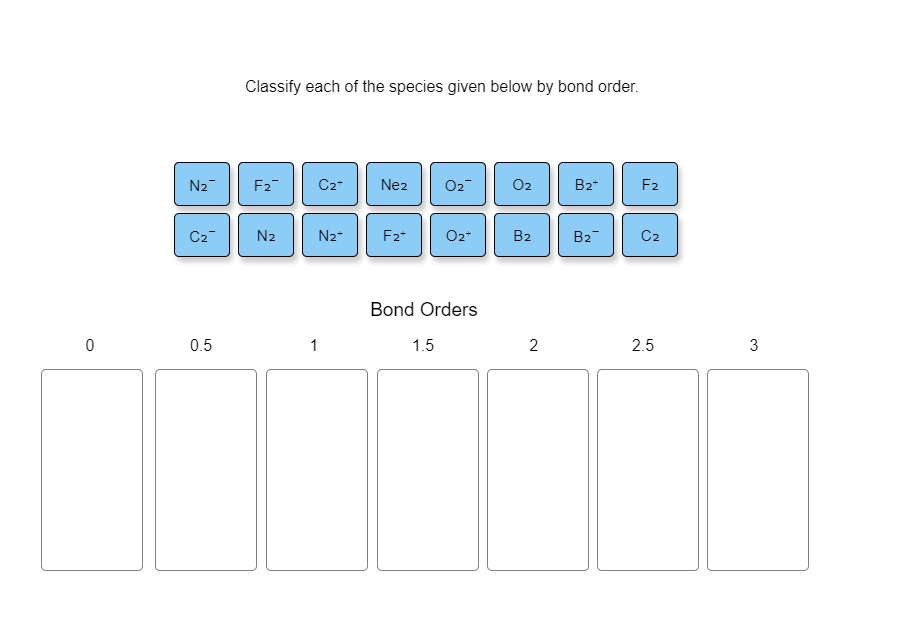
Solved Classify each of the species given below by bond
According to the molecular orbital theory, the bond order of ne2 can be determined. The bond order of ne2 is 0. The bond order of ne2 is 0. The molecular orbital diagram shows the bonding and antibonding. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2.
N2 Molecular Orbital Diagram Bond Order
The bond order of ne2 is 0. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. This means that ne2 does not form a stable bond and does not exist as a molecule. This process.
What is meant by term bond order? Write bond orders for N2, O2?
The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The bond order of ne2+ according to molecular orbital theory is 1.5. The molecular orbital diagram of ne2+ has 8 valence. The bond order of ne2 is 0. This means that ne2 does not form a stable bond and does not.
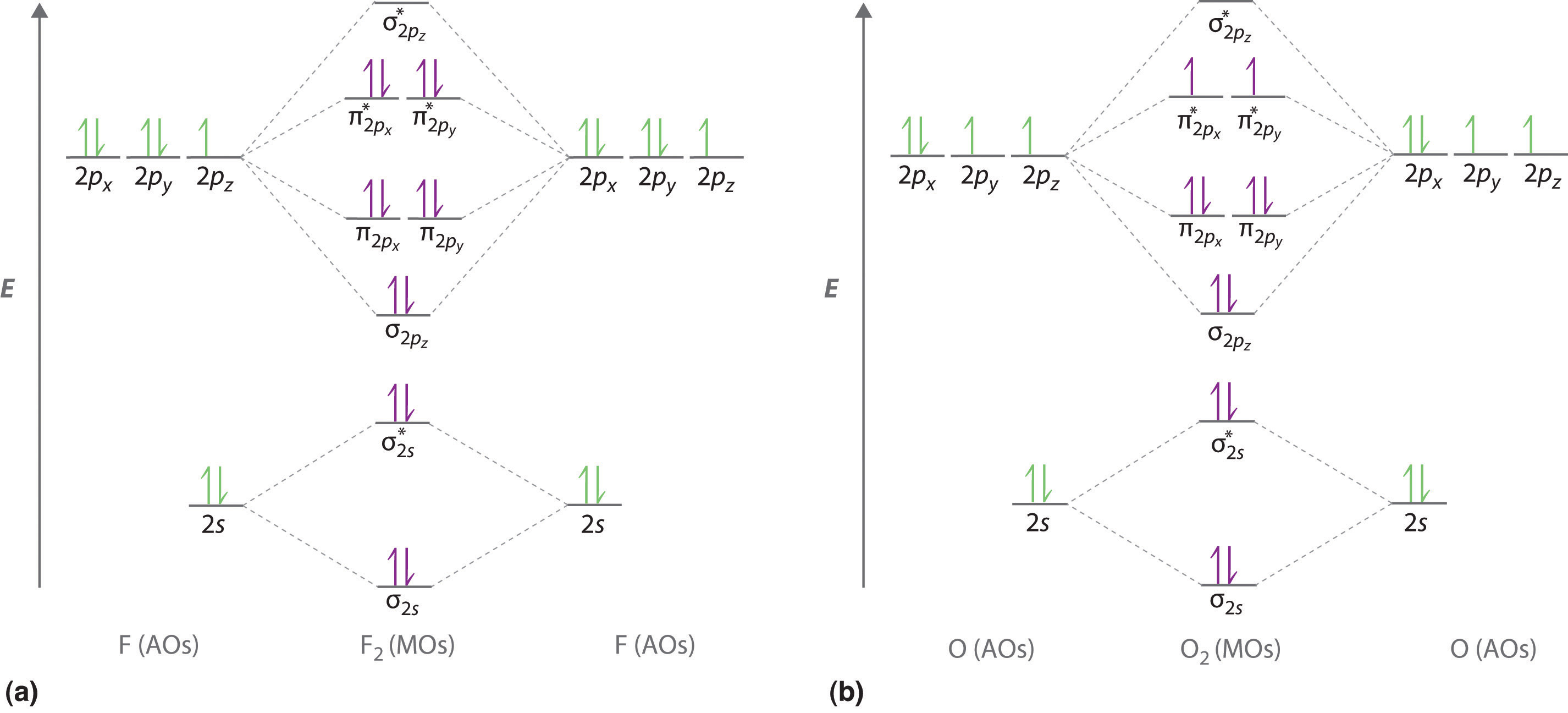
SOLVED Use the molecular orbital diagram below to determine the bond
Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. This process involves the sharing. The molecular orbital diagram of ne2+ has 8 valence. According to the molecular orbital theory, the bond order of ne2 can be determined. The bond order of ne2 is 0.
Bond Order of N2 BrysongroEaton
The molecular orbital diagram shows the bonding and antibonding. This process involves the sharing. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. The bond order of ne2 is 0. This means that ne2 does not form a stable bond and does not exist as a molecule.
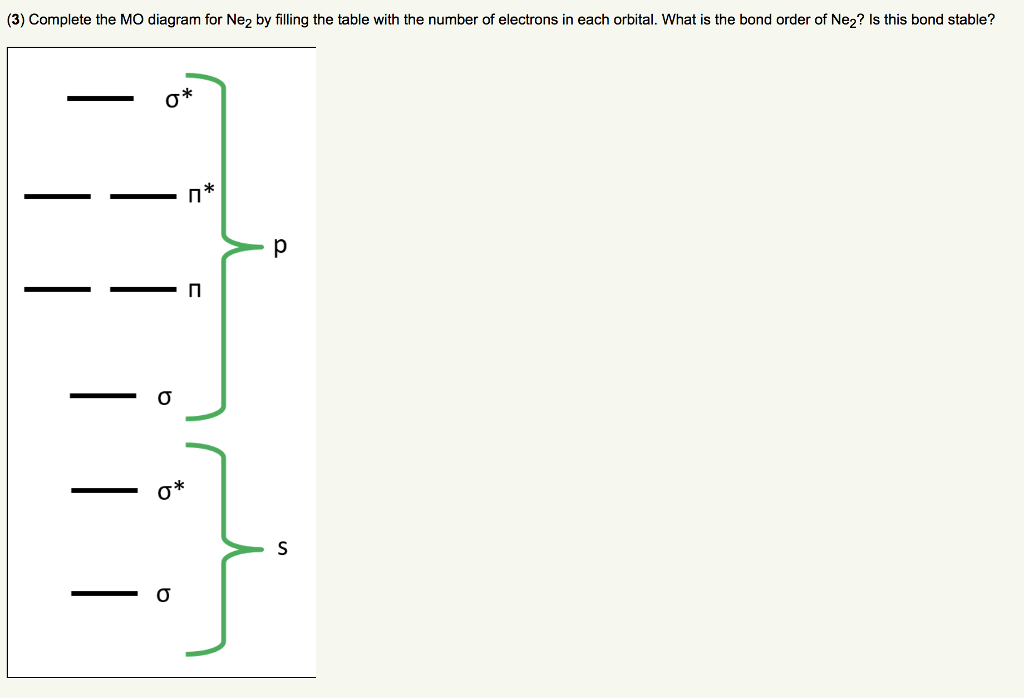
Solved (3) Complete the MO diagram for Ne2 by filling the
The bond order of ne2 is 0. The bond order of ne2 is 0. The bond order of ne2+ according to molecular orbital theory is 1.5. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. According to the molecular orbital theory, the bond order of ne2 can be determined.
What is the bond order of \ce{Ne2}? Quizlet
The molecular orbital diagram shows the bonding and antibonding. The bond order of ne2 is 0. Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. The bond order of ne2+ according to molecular orbital theory is 1.5. This means that ne2 does not form a stable bond and does not exist as a molecule.
The Bond Order Of Ne2 Is 0.
This process involves the sharing. The molecular orbital diagram of ne2+ has 8 valence. The ne2 molecule, also known as the dimer of neon, is formed through the combination of two neon atoms. According to the molecular orbital theory, the bond order of ne2 can be determined.
The Bond Order Of Ne2 Is 0.
Ne2 is a diatomic molecule of two neon atoms with a bond order of 2. The bond order of ne2+ according to molecular orbital theory is 1.5. The molecular orbital diagram shows the bonding and antibonding. This means that ne2 does not form a stable bond and does not exist as a molecule.