What Is The Concentration Of The Unknown H2So4 Solution - Express your answer using four significant figures., a 5.00 ml sample of an. What is the concentration of the unknown h2so4 solution? 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to.
Express your answer using four significant figures., a 5.00 ml sample of an. What is the concentration of the unknown h2so4 solution? 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to.
To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to. 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. What is the concentration of the unknown h2so4 solution? To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. Express your answer using four significant figures., a 5.00 ml sample of an.
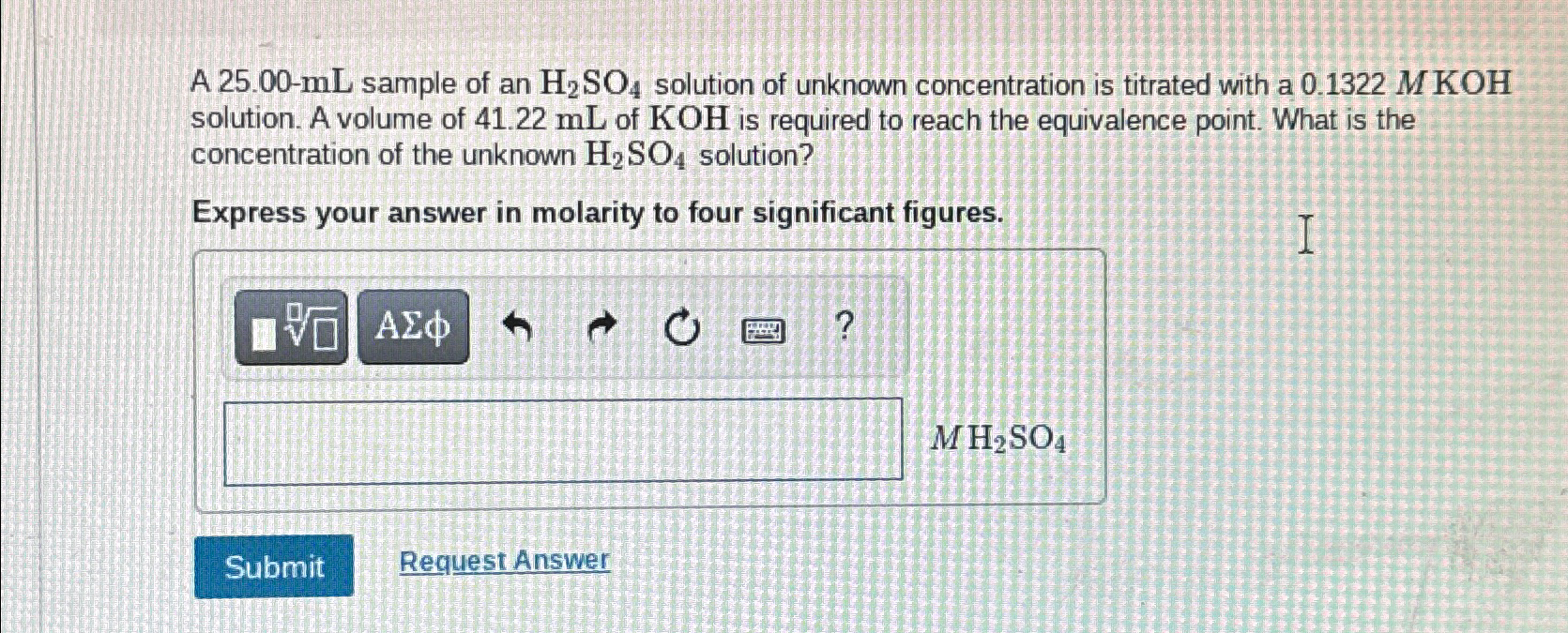
Solved A 25.00mL sample of an H2SO4 solution of unknown
What is the concentration of the unknown h2so4 solution? Express your answer using four significant figures., a 5.00 ml sample of an. To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the.
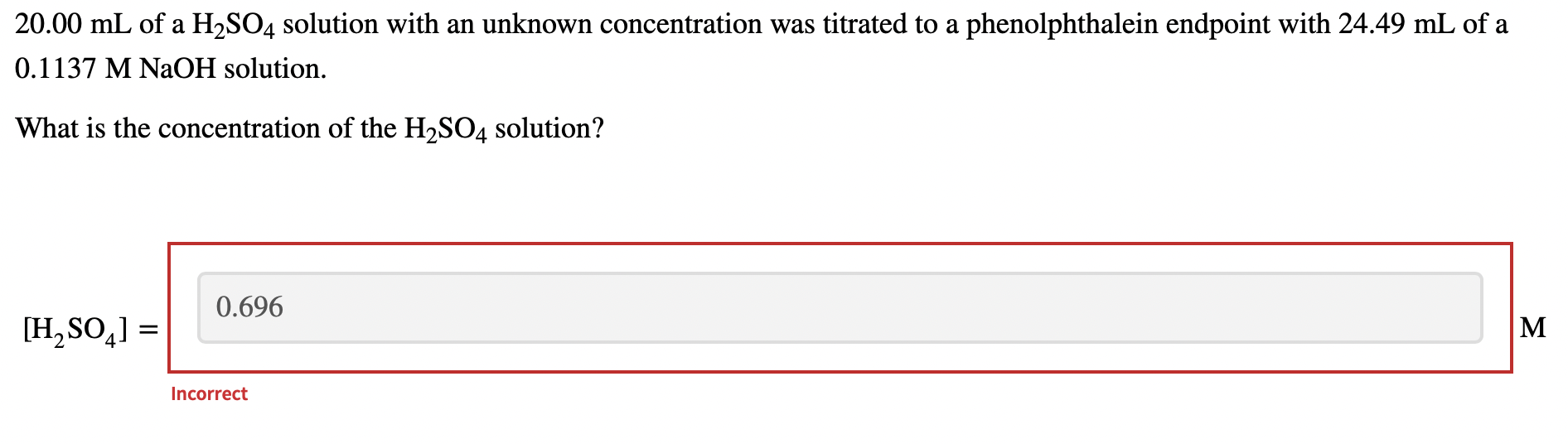
Solved 20.00 mL of a H2SO4 solution with an unknown
To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To find the concentration of an unknown solution, you would typically use a titration method involving a solution.
Solved What is the concentration of the unknown H2SO4
What is the concentration of the unknown h2so4 solution? To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. Express your answer using four significant figures., a 5.00.
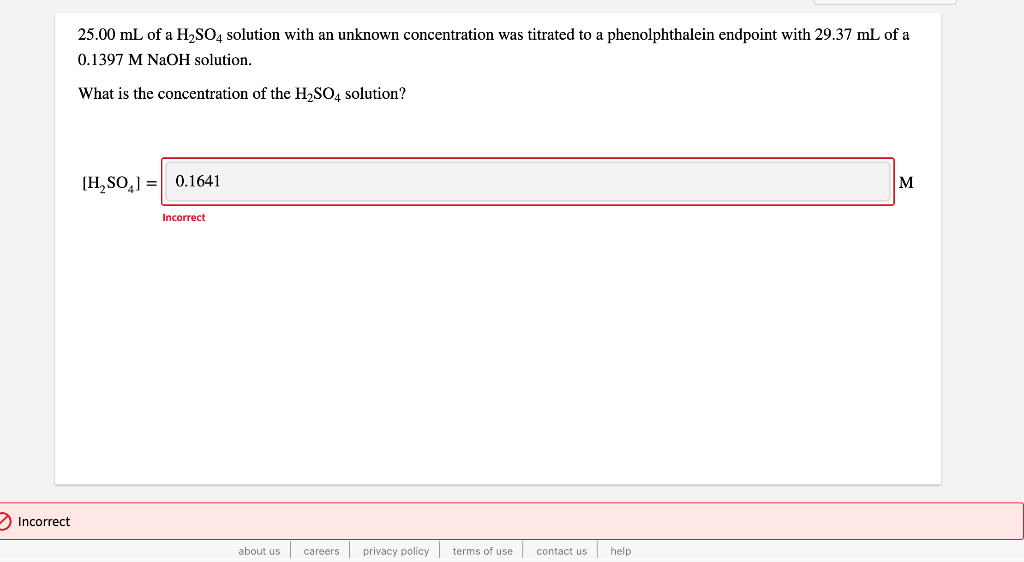
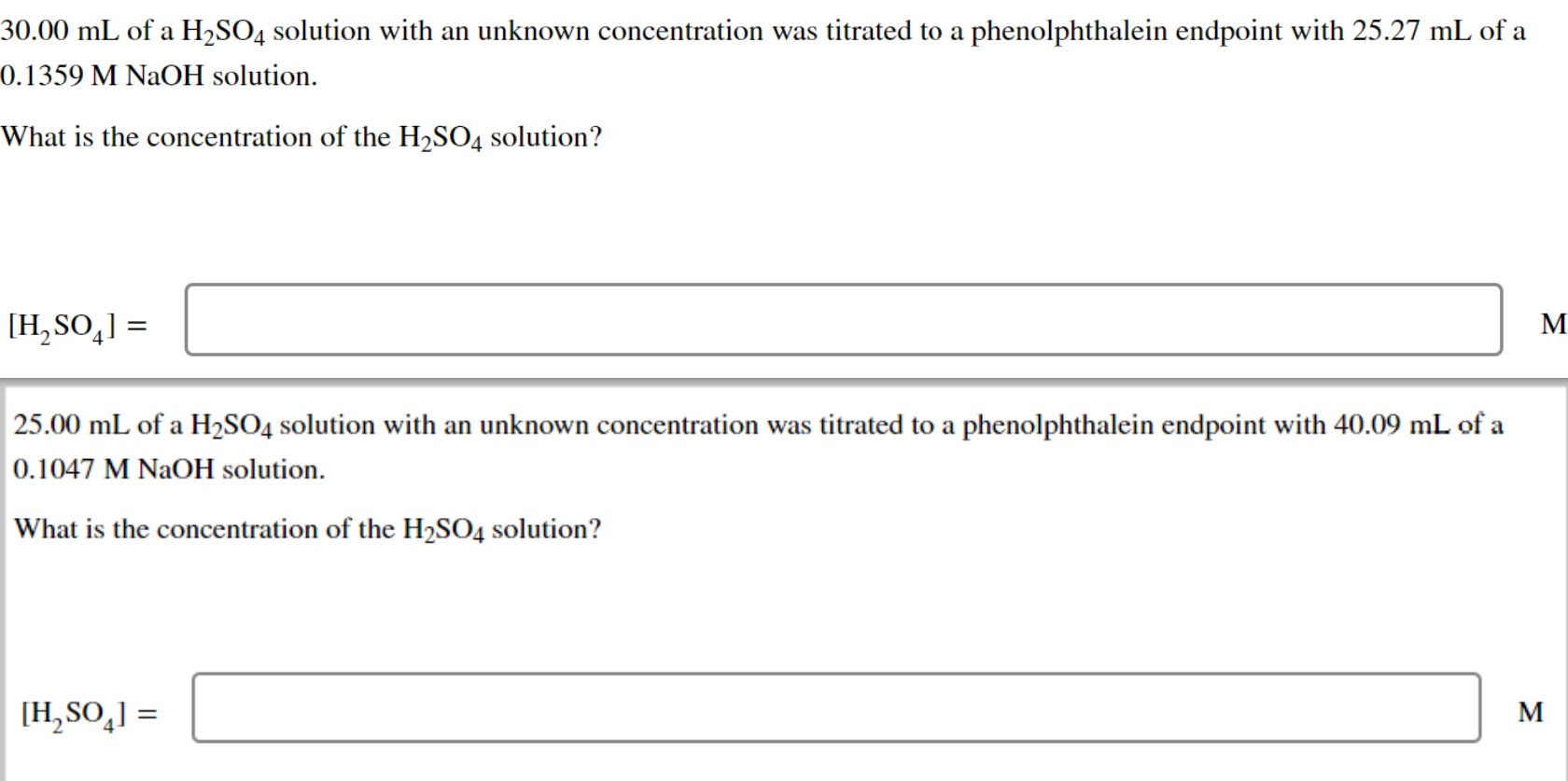
Solved 25.00 mL of a H2SO4 solution with an unknown
What is the concentration of the unknown h2so4 solution? 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. Express your answer using four significant figures., a 5.00.
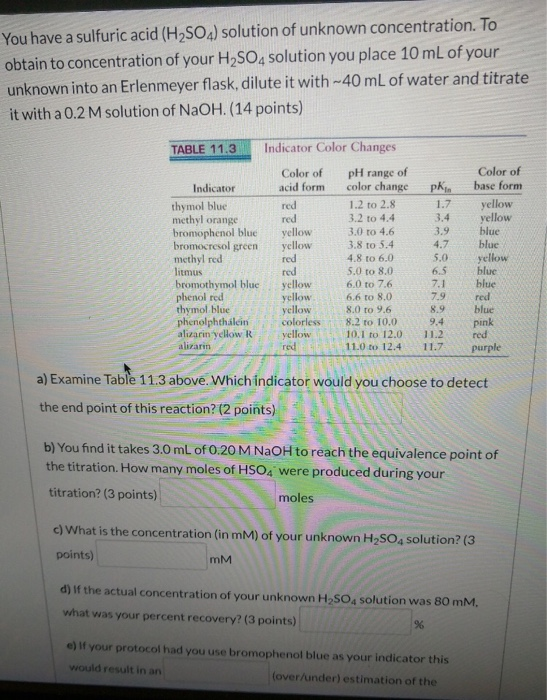
Solved You have a sulfuric acid (H2SO4) solution of unknown
What is the concentration of the unknown h2so4 solution? Express your answer using four significant figures., a 5.00 ml sample of an. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint.
Solved 30.00 mL of a H2SO4 solution with an unknown
Express your answer using four significant figures., a 5.00 ml sample of an. What is the concentration of the unknown h2so4 solution? To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to. 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to.
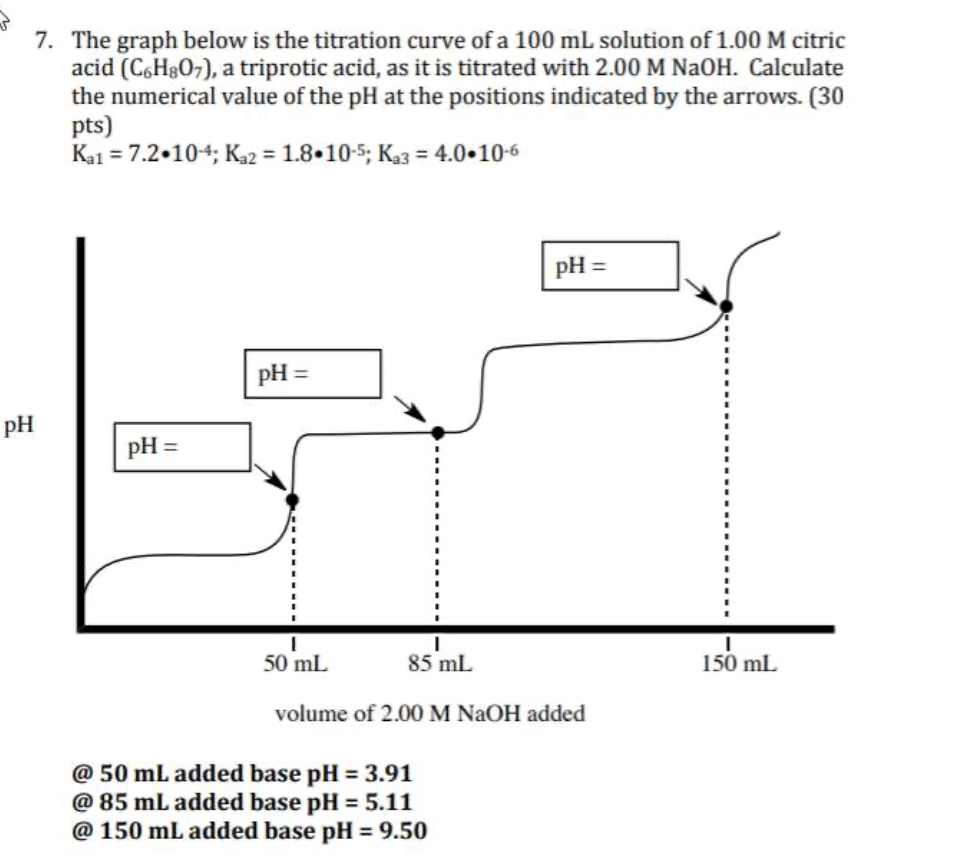
Solved 5 9. A 100 mL solution of unknown H2SO4 concentration
To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to. 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. What is the concentration of the unknown h2so4 solution? To determine the concentration of the.
Solved 5 9. A 100 mL solution of unknown H2SO4 concentration
20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. What is the concentration of the unknown h2so4 solution? To find the concentration of an unknown solution, you.
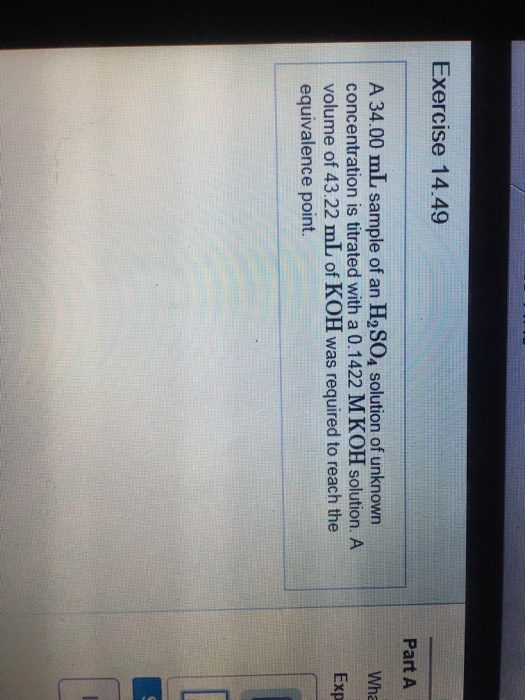
Solved A 34.00 mL sample of an H_2SO_4 solution of unknown
Express your answer using four significant figures., a 5.00 ml sample of an. What is the concentration of the unknown h2so4 solution? 20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To find the concentration of an unknown solution, you would typically use a titration method.
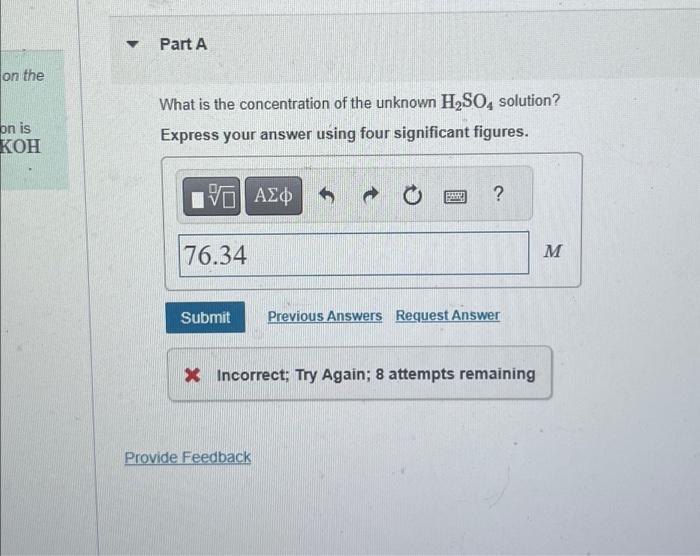
Solved Part A What is the concentration of the unknown H2SO4
20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. Express your answer using four significant figures., a 5.00 ml sample of an. What is the concentration of.
What Is The Concentration Of The Unknown H2So4 Solution?
20.0020.00 ml of a h 2 so 4 solution with an unknown concentration was titrated to a phenolphthalein endpoint with 35.7135.71 ml of. To determine the concentration of the unknown h2so4 solution, we can use the stoichiometry of the reaction between h2so4. To find the concentration of an unknown solution, you would typically use a titration method involving a solution of known concentration to. Express your answer using four significant figures., a 5.00 ml sample of an.