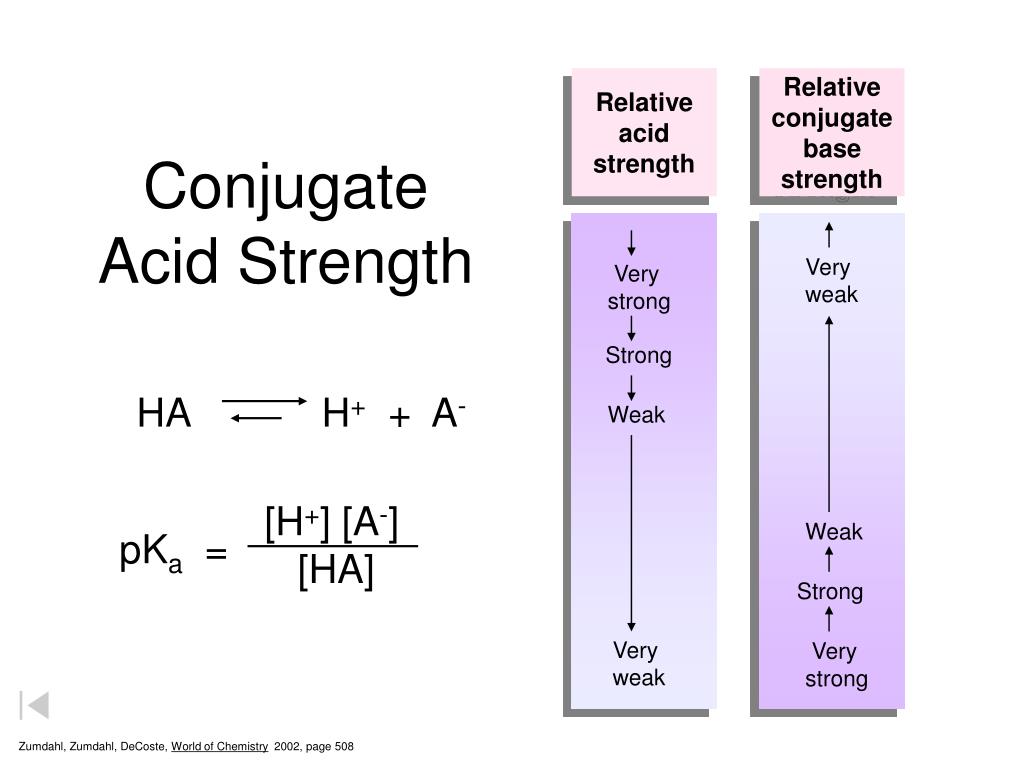
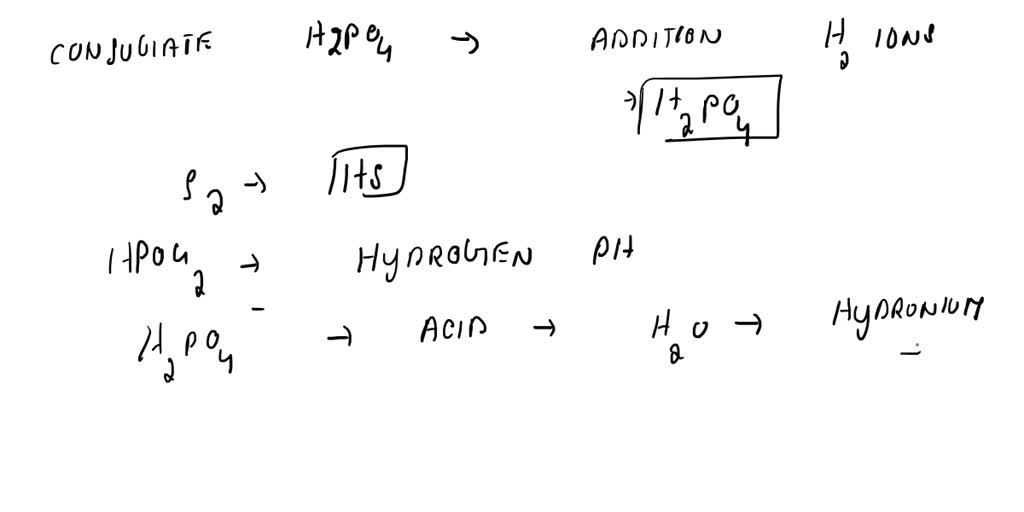
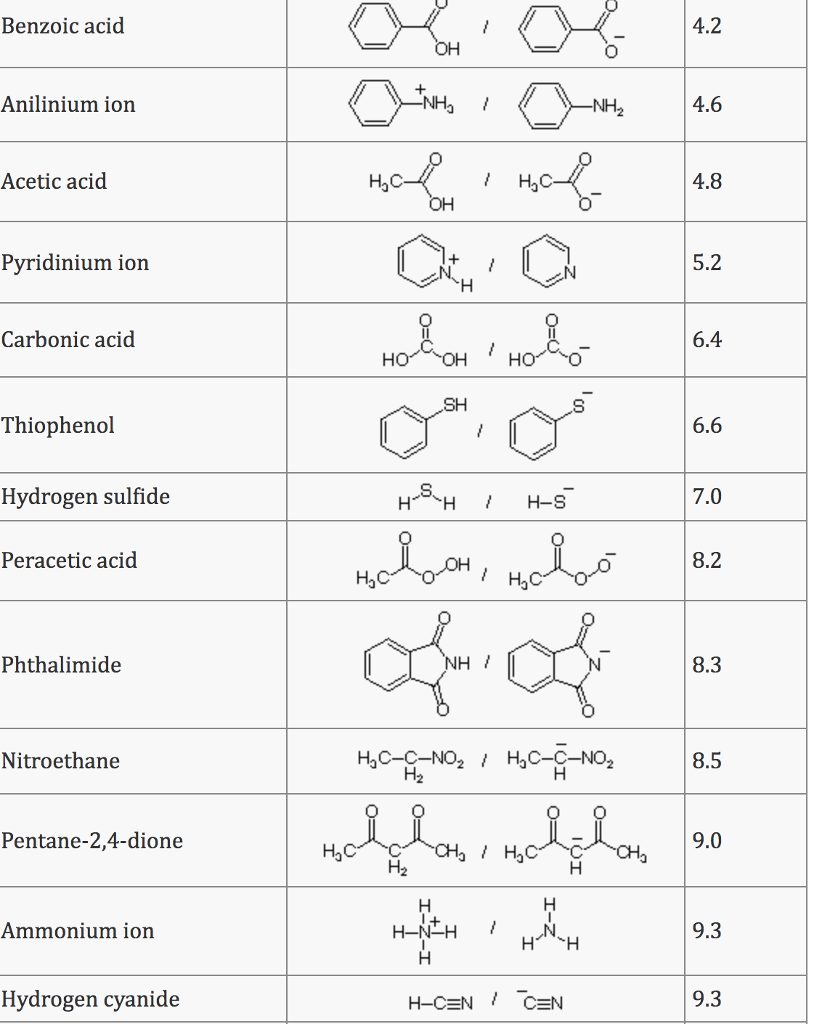
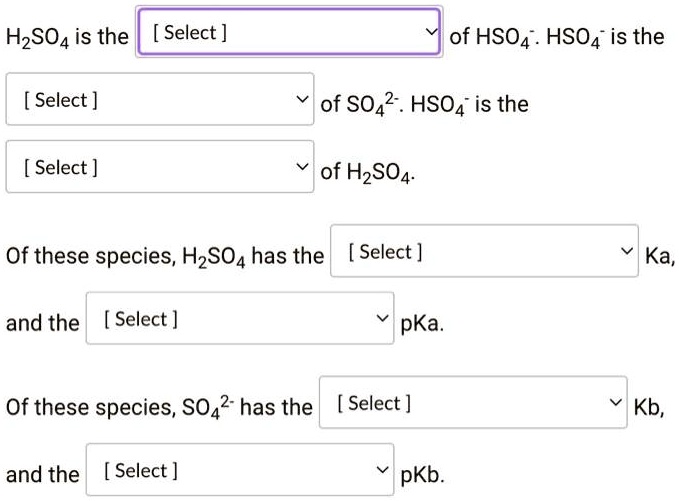
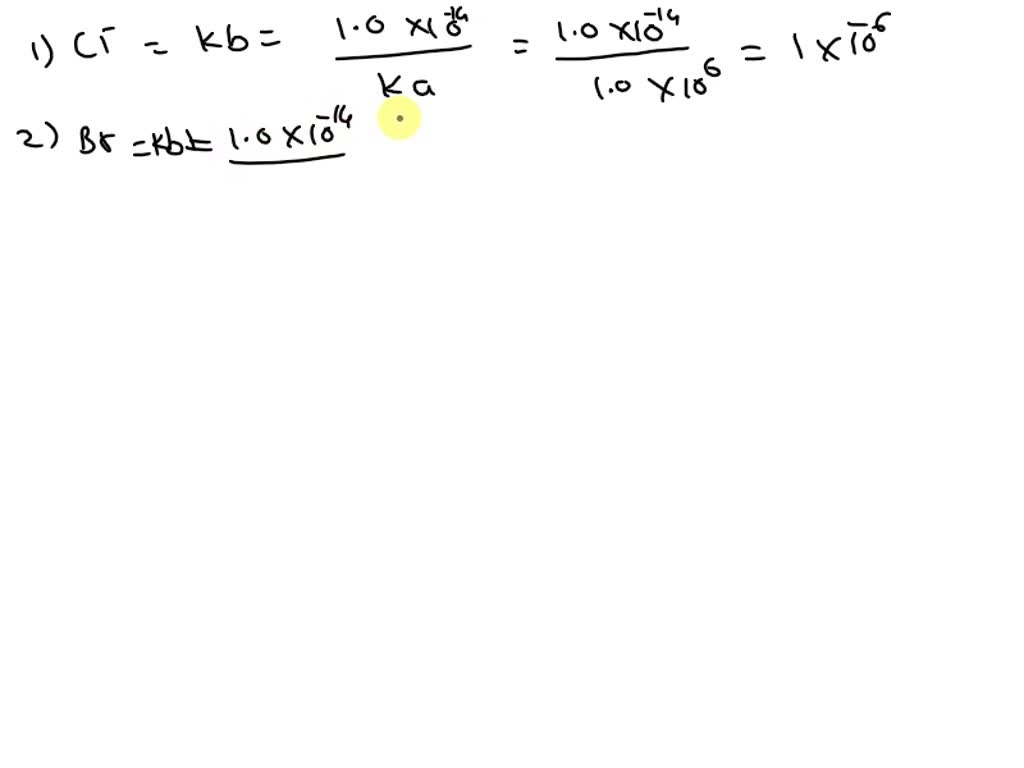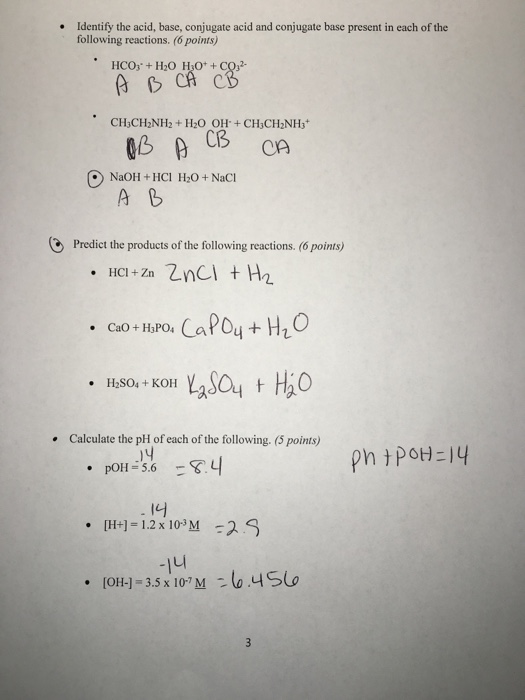What Is The Conjugate Acid Of So42 - The conjugate acid of sulfate is. Removing one h + gives you its conjugate base. Identify the formula for the conjugate acid of so42−. Adding one h + to anything gives you its conjugate acid. The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. To identify the acids associated with the given conjugate bases, we can follow this logic: Your solution’s ready to go! Enhanced with ai, our expert help has broken down your.
Removing one h + gives you its conjugate base. The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. To identify the acids associated with the given conjugate bases, we can follow this logic: Enhanced with ai, our expert help has broken down your. The conjugate acid of sulfate is. Your solution’s ready to go! Identify the formula for the conjugate acid of so42−. Adding one h + to anything gives you its conjugate acid.
Adding one h + to anything gives you its conjugate acid. The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. Enhanced with ai, our expert help has broken down your. Removing one h + gives you its conjugate base. The conjugate acid of sulfate is. To identify the acids associated with the given conjugate bases, we can follow this logic: Identify the formula for the conjugate acid of so42−. Your solution’s ready to go!
PPT Strengths of Conjugate AcidBase Pairs PowerPoint Presentation
Adding one h + to anything gives you its conjugate acid. Removing one h + gives you its conjugate base. Your solution’s ready to go! The conjugate acid of sulfate is. Enhanced with ai, our expert help has broken down your.
SOLVED The formula for the conjugate acid of S2 is ??The formula for
Removing one h + gives you its conjugate base. Adding one h + to anything gives you its conjugate acid. The conjugate acid of sulfate is. To identify the acids associated with the given conjugate bases, we can follow this logic: The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton.
Solved 12) (8 marks) What is the a) conjugate acid of SO42
Your solution’s ready to go! The conjugate acid of sulfate is. Enhanced with ai, our expert help has broken down your. Removing one h + gives you its conjugate base. To identify the acids associated with the given conjugate bases, we can follow this logic:
Solved Name Acid / Conjugate base pKa Sulfuric acid ?? ??
To identify the acids associated with the given conjugate bases, we can follow this logic: The conjugate acid of sulfate is. Enhanced with ai, our expert help has broken down your. Identify the formula for the conjugate acid of so42−. Removing one h + gives you its conjugate base.
SOLVED 'Write the formula of the conjugate acid of the BronstedLowry
Removing one h + gives you its conjugate base. Enhanced with ai, our expert help has broken down your. The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. Identify the formula for the conjugate acid of so42−. The conjugate acid of sulfate is.
SOLVED Texts dropdown menu 1. conjugate acid or conjugate base 2
The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. Adding one h + to anything gives you its conjugate acid. Removing one h + gives you its conjugate base. To identify the acids associated with the given conjugate bases, we can follow this logic: Identify the formula for the conjugate acid of.
Conjugate Acids And Bases Acid Base Equilibria MCAT Content
Removing one h + gives you its conjugate base. Adding one h + to anything gives you its conjugate acid. The conjugate acid of sulfate is. To identify the acids associated with the given conjugate bases, we can follow this logic: Enhanced with ai, our expert help has broken down your.
Solved 1. Give the conjugate base of each of the following
The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. Removing one h + gives you its conjugate base. To identify the acids associated with the given conjugate bases, we can follow this logic: Your solution’s ready to go! The conjugate acid of sulfate is.
SOLVED Texts dropdown menu 1. conjugate acid or conjugate base 2
Removing one h + gives you its conjugate base. Identify the formula for the conjugate acid of so42−. To identify the acids associated with the given conjugate bases, we can follow this logic: Your solution’s ready to go! The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton.
Solved Identify the acid, base, conjugate acid and conjugate
The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton. Removing one h + gives you its conjugate base. To identify the acids associated with the given conjugate bases, we can follow this logic: Enhanced with ai, our expert help has broken down your. The conjugate acid of sulfate is.
Removing One H + Gives You Its Conjugate Base.
To identify the acids associated with the given conjugate bases, we can follow this logic: Enhanced with ai, our expert help has broken down your. Your solution’s ready to go! The conjugate acid of so4²− is hso4− because it forms when the sulfate ion accepts a proton.
Identify The Formula For The Conjugate Acid Of So42−.
The conjugate acid of sulfate is. Adding one h + to anything gives you its conjugate acid.