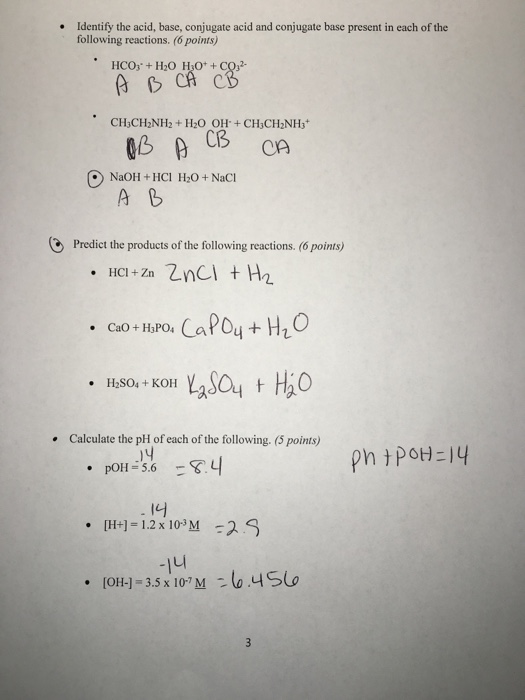What Is The Conjugate Base Of Ch3Nh2 - It can act as a base by accepting a proton from. From the definition given above, ch3nh2 is a base. Therefore, the conjugate acid of ch3nh2 is ch3nh3+. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Identify the conjugate base by determining what turns into after donating a proton (). A conjugate base is formed when a proton is removed from an acid. Ch3nh2, also known as methylamine, is a weak base and not an acid. Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. The conjugate base is determined by “removing” h(1+) from the formula, so the conjugate base of ch3nh2 is:
When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Ch3nh2, also known as methylamine, is a weak base and not an acid. Identify the conjugate base by determining what turns into after donating a proton (). Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. The conjugate base is determined by “removing” h(1+) from the formula, so the conjugate base of ch3nh2 is: From the definition given above, ch3nh2 is a base. Therefore, the conjugate acid of ch3nh2 is ch3nh3+. It can act as a base by accepting a proton from. A conjugate base is formed when a proton is removed from an acid.
Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. Therefore, the conjugate acid of ch3nh2 is ch3nh3+. The conjugate base is determined by “removing” h(1+) from the formula, so the conjugate base of ch3nh2 is: Ch3nh2, also known as methylamine, is a weak base and not an acid. From the definition given above, ch3nh2 is a base. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Identify the conjugate base by determining what turns into after donating a proton (). It can act as a base by accepting a proton from. A conjugate base is formed when a proton is removed from an acid.
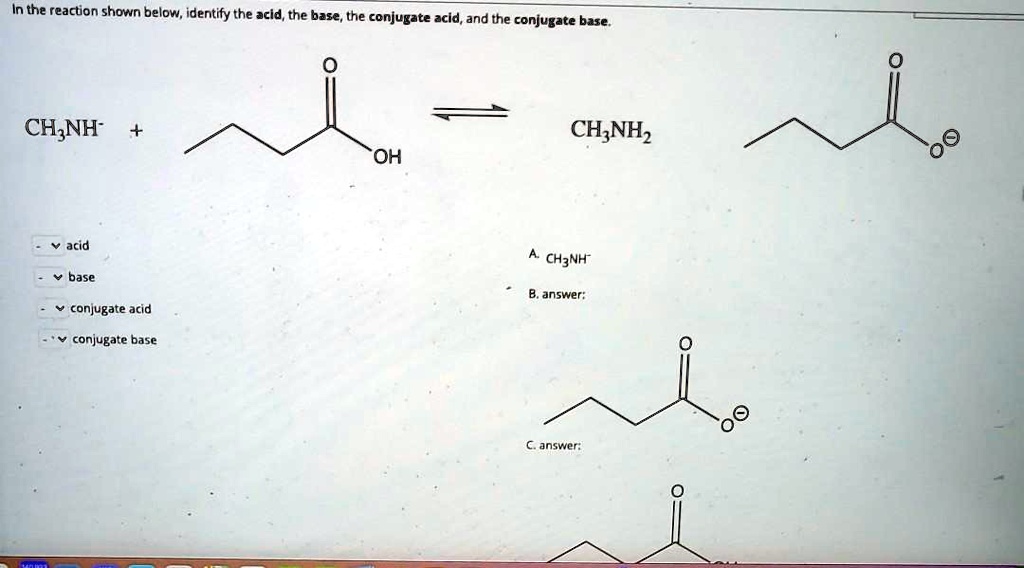
SOLVED In the reaction shown below, identify the acid; the base, the
Ch3nh2, also known as methylamine, is a weak base and not an acid. A conjugate base is formed when a proton is removed from an acid. The conjugate base is determined by “removing” h(1+) from the formula, so the conjugate base of ch3nh2 is: Identify the conjugate base by determining what turns into after donating a proton (). From the.
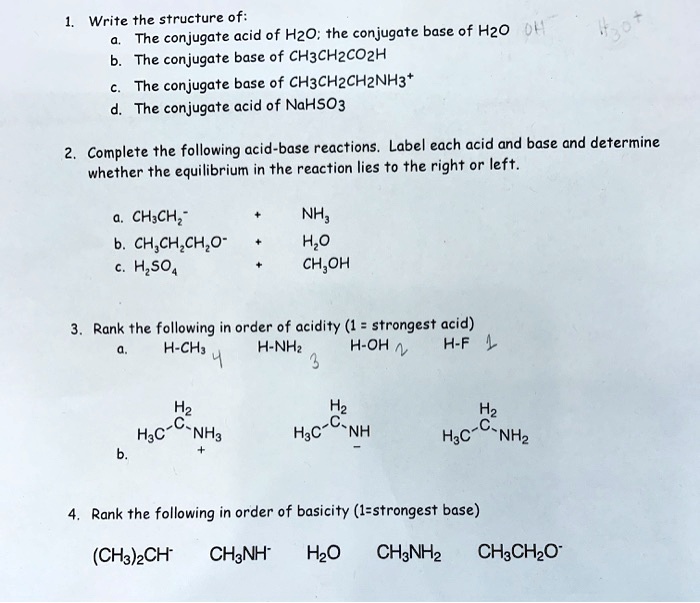
SOLVED Write the structure of acid of H2O; the conjugate base of H2O
Therefore, the conjugate acid of ch3nh2 is ch3nh3+. Identify the conjugate base by determining what turns into after donating a proton (). Ch3nh2, also known as methylamine, is a weak base and not an acid. A conjugate base is formed when a proton is removed from an acid. The conjugate base is determined by “removing” h(1+) from the formula, so.
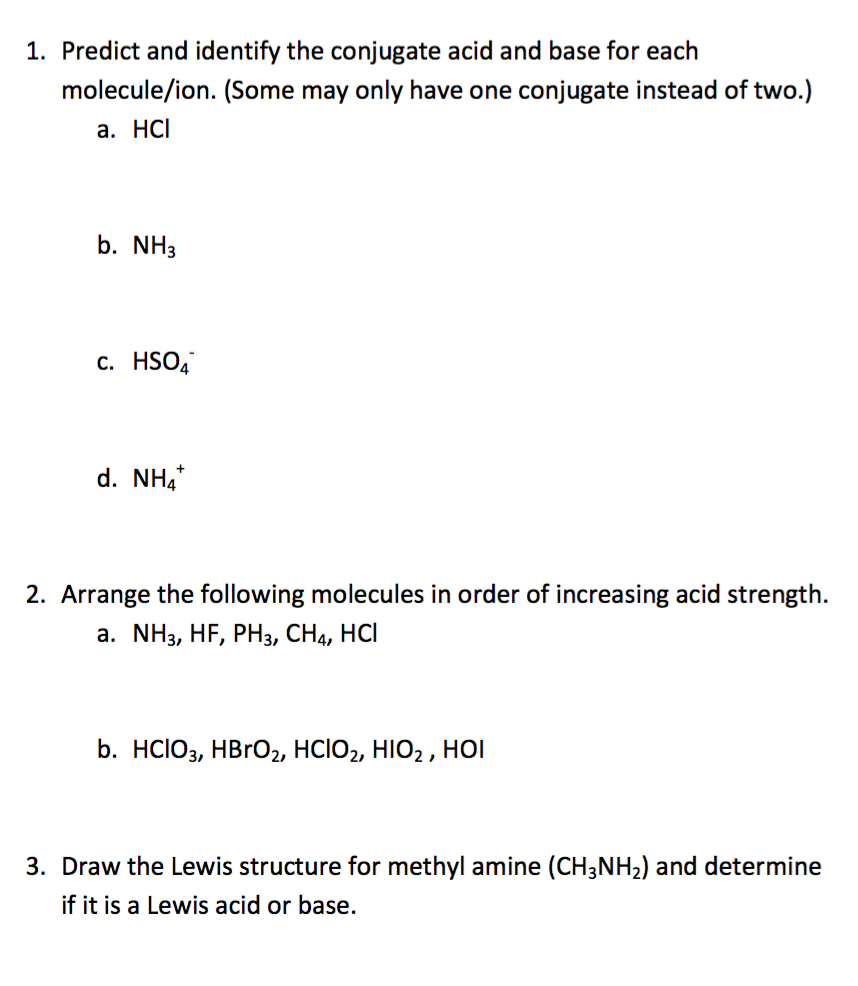
Predict and identify the conjugate acid and base for
It can act as a base by accepting a proton from. Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. From the definition given above, ch3nh2 is a base. A conjugate base is formed when a proton is removed from an acid. The conjugate base is.
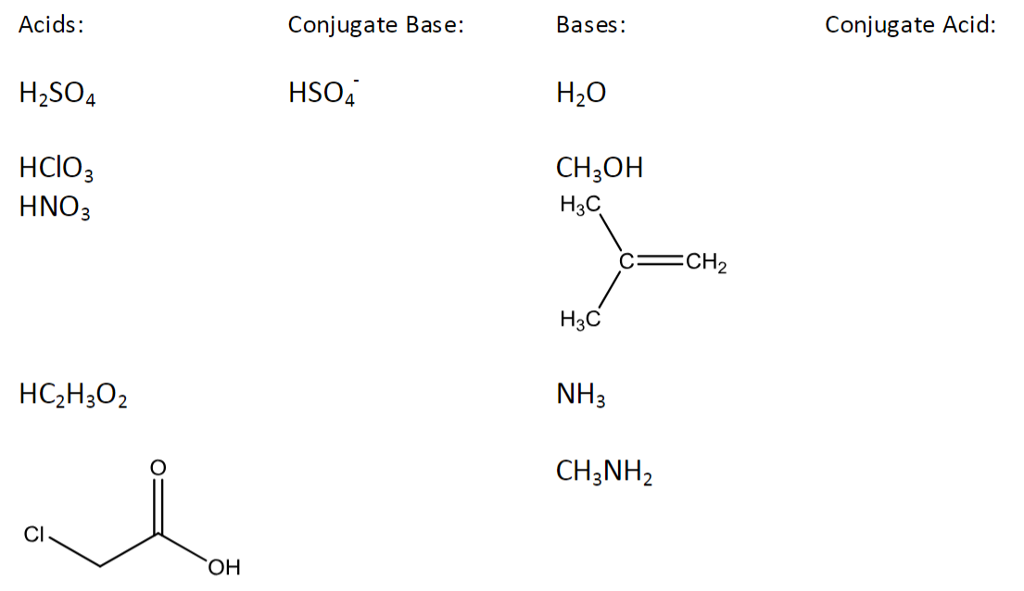
Solved Bases Conjugate Acid Conjugate Base Acids H2SO4
Therefore, the conjugate acid of ch3nh2 is ch3nh3+. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. The conjugate base is determined by “removing” h(1+) from the formula, so.
Conjugate Base Of H3po4 Asking List
Therefore, the conjugate acid of ch3nh2 is ch3nh3+. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Identify the conjugate base by determining what turns into after donating a proton (). From the definition given above, ch3nh2 is a base. The conjugate base is determined by “removing” h(1+) from the formula, so.
SOLVED 8. Identify the acid, base, conjugate acid, and conjugate base
Ch3nh2, also known as methylamine, is a weak base and not an acid. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. The conjugate base is determined by “removing” h(1+) from the formula, so the conjugate base of ch3nh2 is: Study with quizlet and memorize flashcards containing terms like what is the.
Solved Identify the acid, base, conjugate acid and conjugate
Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. Ch3nh2, also known as methylamine, is a weak base and not an acid. Identify the conjugate base by determining what turns into after donating a proton (). When a base ch3ch2 accept proton it form its conjugate.
Solved Draw the conjugate base of the following Acids
Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a. The conjugate base is determined by “removing” h(1+) from the formula, so the conjugate base of ch3nh2 is: From the definition given above, ch3nh2 is a base. Therefore, the conjugate acid of ch3nh2 is ch3nh3+. It can.
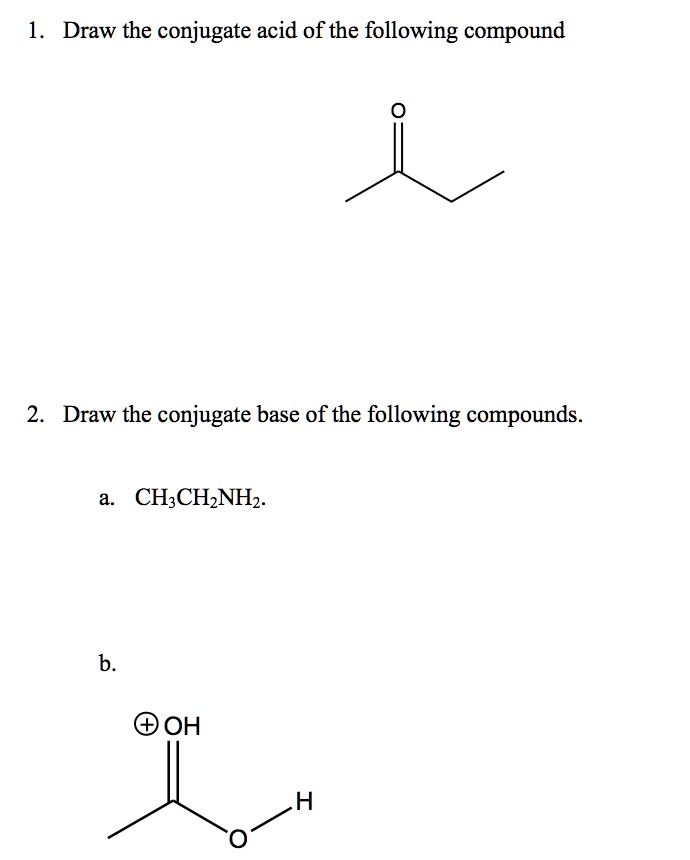
SOLVED Draw the conjugate acid of the following compound CH3CH2NH2
A conjugate base is formed when a proton is removed from an acid. It can act as a base by accepting a proton from. From the definition given above, ch3nh2 is a base. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Therefore, the conjugate acid of ch3nh2 is ch3nh3+.
Answered 5. For each reaction, identify the… bartleby
From the definition given above, ch3nh2 is a base. When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. Ch3nh2, also known as methylamine, is a weak base and not an acid. Identify the conjugate base by determining what turns into after donating a proton (). The conjugate base is determined by “removing”.
A Conjugate Base Is Formed When A Proton Is Removed From An Acid.
From the definition given above, ch3nh2 is a base. Ch3nh2, also known as methylamine, is a weak base and not an acid. Therefore, the conjugate acid of ch3nh2 is ch3nh3+. Identify the conjugate base by determining what turns into after donating a proton ().
The Conjugate Base Is Determined By “Removing” H(1+) From The Formula, So The Conjugate Base Of Ch3Nh2 Is:
When a base ch3ch2 accept proton it form its conjugate acid which known as methyl ammonium ion. It can act as a base by accepting a proton from. Study with quizlet and memorize flashcards containing terms like what is the conjugate base of ch3nh2?, which of the following is not a.