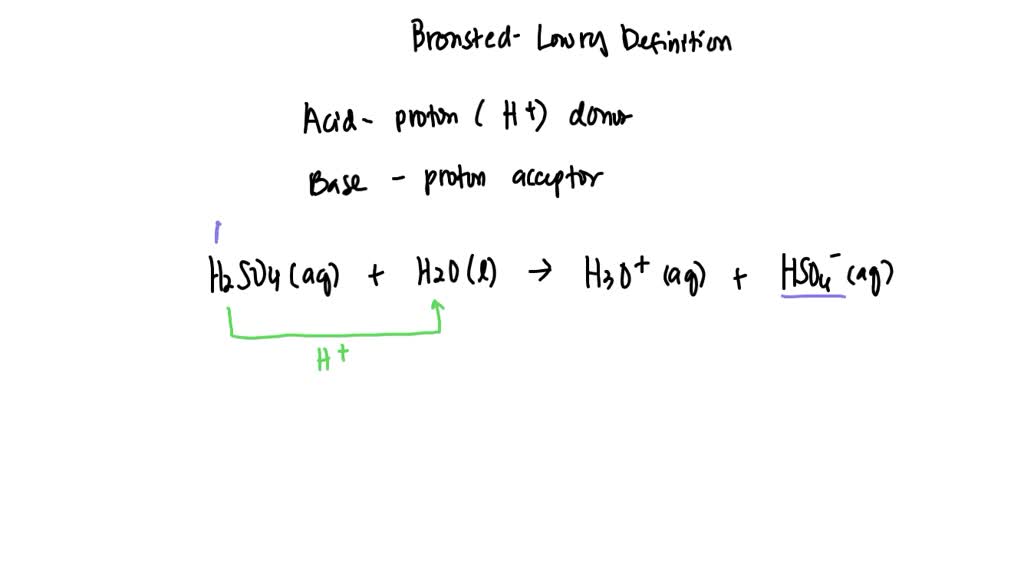
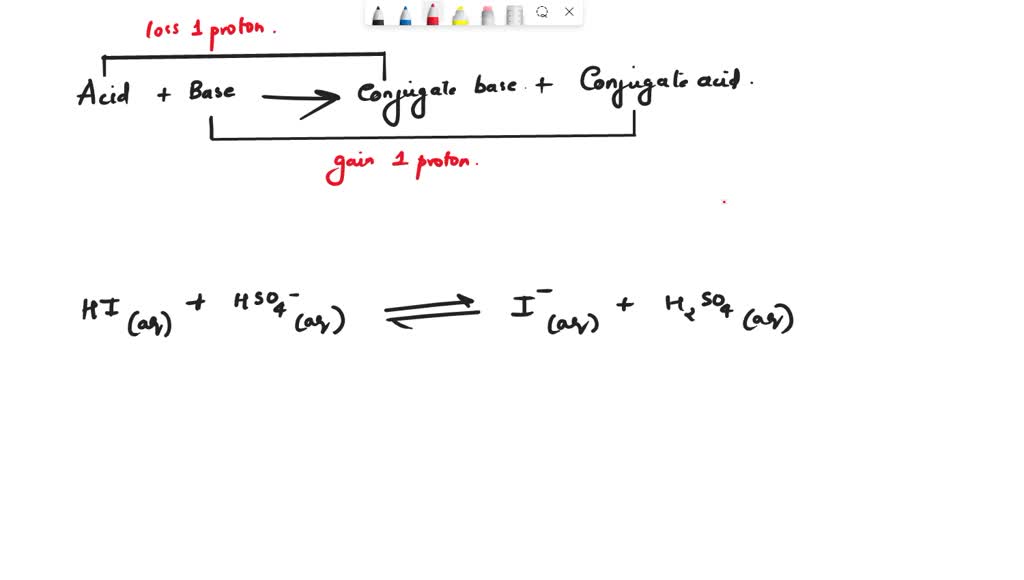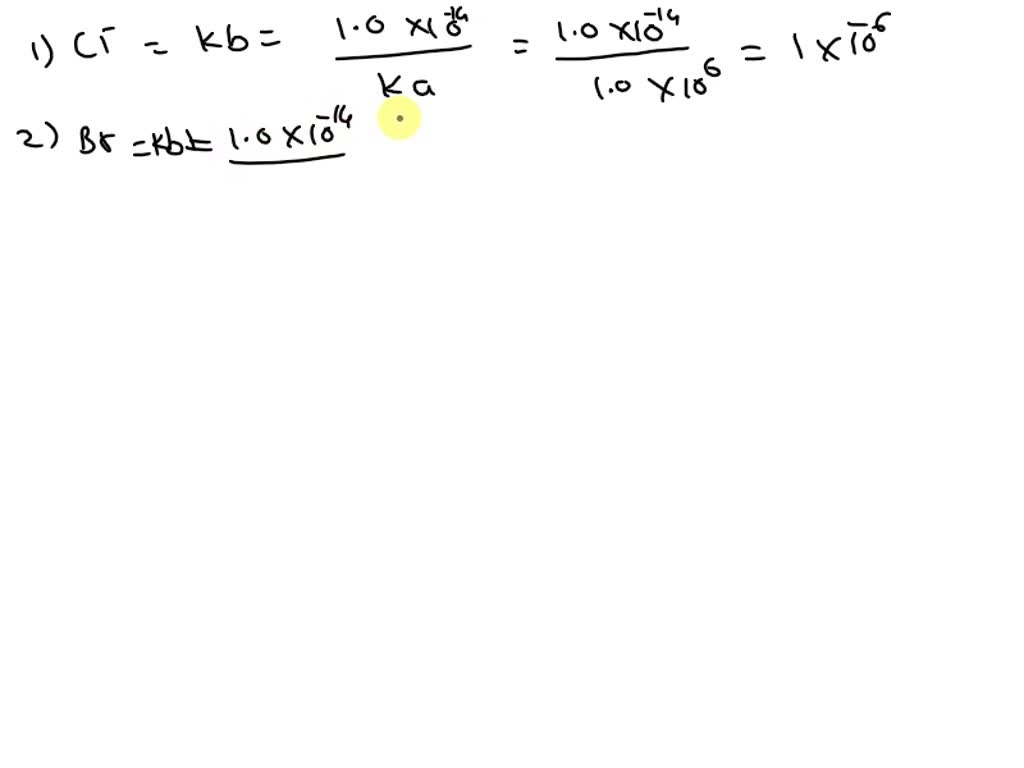What Is The Conjugate Base Of H2So4 - The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. The chemical reaction for the above. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 There are 2 steps to solve this one. To find the conjugate base of an acid, you remove a proton (h+) from it. Sulfuric acid (h2so4) will lose a proton to form its conjugate base. The conjugate base of h 2 s o 4 in the following reaction is: The conjugate base of a substance is formed by removing a proton (h a +) from it.
The conjugate base of h 2 s o 4 in the following reaction is: The chemical reaction for the above. To find the conjugate base of an acid, you remove a proton (h+) from it. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 Sulfuric acid (h2so4) will lose a proton to form its conjugate base. The conjugate base of a substance is formed by removing a proton (h a +) from it. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. There are 2 steps to solve this one.
The conjugate base of a substance is formed by removing a proton (h a +) from it. To find the conjugate base of an acid, you remove a proton (h+) from it. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 The chemical reaction for the above. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. There are 2 steps to solve this one. The conjugate base of h 2 s o 4 in the following reaction is: Sulfuric acid (h2so4) will lose a proton to form its conjugate base.
H2SO4(aq)+H2O(l)←→H3O+(aq)+HSO4−(aq) acid H2SO4 conjugate base HSO4−
H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 The conjugate base of a substance is formed by removing a proton (h a +) from it. The conjugate base of h 2 s o 4 in the following reaction is: Sulfuric acid (h2so4) will lose a proton to form.
SOLUTION Conjugate Acid and Conjugate Base pKa Chart Studypool
There are 2 steps to solve this one. The conjugate base of h 2 s o 4 in the following reaction is: The chemical reaction for the above. Sulfuric acid (h2so4) will lose a proton to form its conjugate base. To find the conjugate base of an acid, you remove a proton (h+) from it.
SOLVED Identify the conjugate base for each acid. * conjugate base of
There are 2 steps to solve this one. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 To find the conjugate base of an acid, you remove a proton (h+) from it. Sulfuric acid (h2so4) will lose a proton to form its conjugate base. The chemical reaction for the.
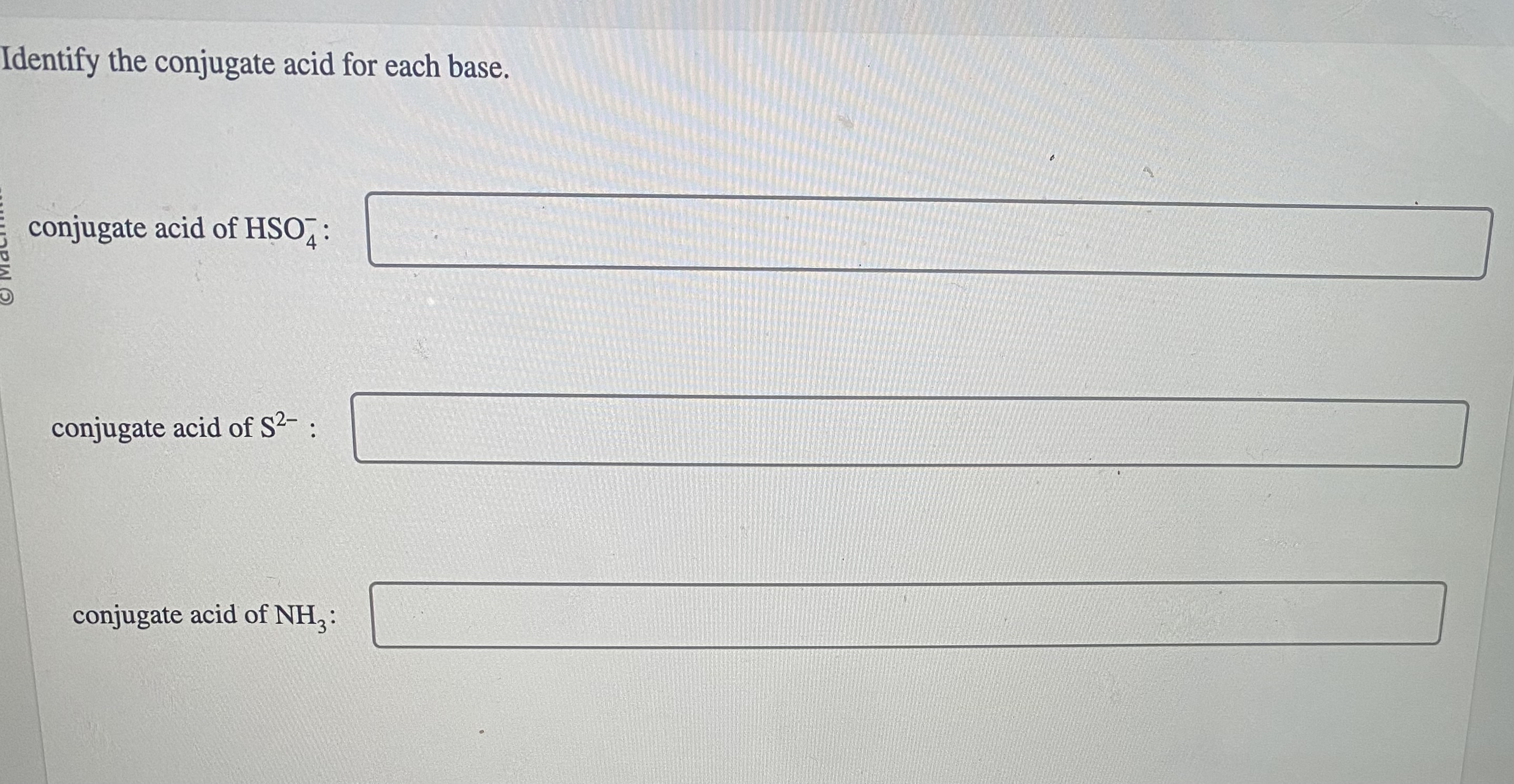
Solved Identify the conjugate acid for each base. conjugate
The chemical reaction for the above. Sulfuric acid (h2so4) will lose a proton to form its conjugate base. There are 2 steps to solve this one. The conjugate base of h 2 s o 4 in the following reaction is: To find the conjugate base of an acid, you remove a proton (h+) from it.
SOLVED Identify the conjugate base for each acid. conjugate base of
The conjugate base of a substance is formed by removing a proton (h a +) from it. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 There are 2 steps to solve this one. The chemical reaction for the above. The conjugate base of a strong acid is a.
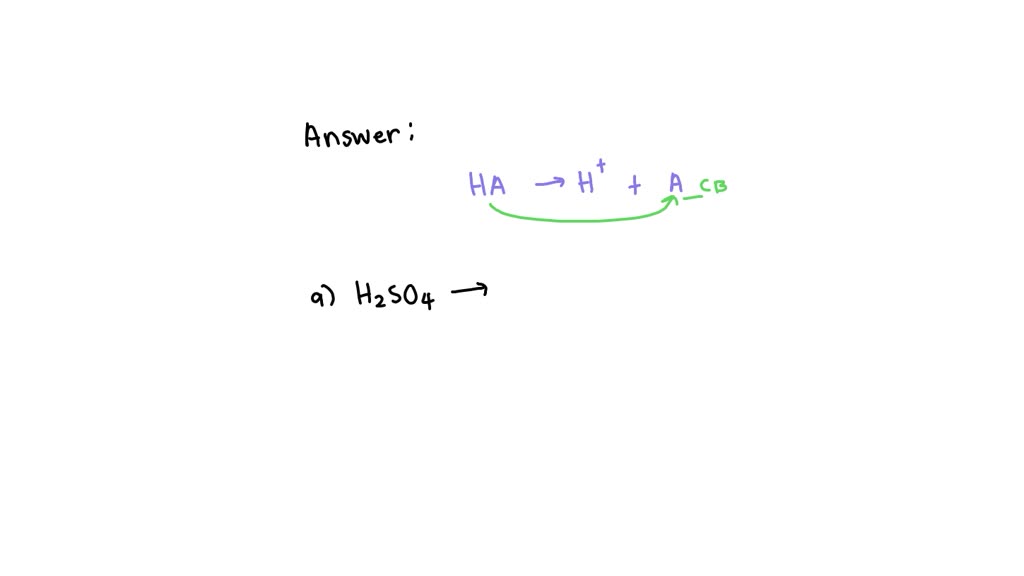
Solved Give The Conjugate Base For Each Compound Below. A...
The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. The chemical reaction for the above. To find the conjugate base of an acid, you remove a proton (h+) from it. The conjugate base of h 2 s o 4 in the following reaction is:.
Conjugate Acid Base Chart
The conjugate base of h 2 s o 4 in the following reaction is: To find the conjugate base of an acid, you remove a proton (h+) from it. There are 2 steps to solve this one. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a.
SOLUTION Conjugate Acid and Conjugate Base pKa Chart Studypool
To find the conjugate base of an acid, you remove a proton (h+) from it. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 The conjugate base of a substance is formed by removing a proton (h a +) from it. The chemical reaction for the above. The conjugate.
SOLVED Texts dropdown menu 1. conjugate acid or conjugate base 2
To find the conjugate base of an acid, you remove a proton (h+) from it. The conjugate base of h 2 s o 4 in the following reaction is: H 2 s o 4 + h 2 o → h 3 o + + h s o − 4 Sulfuric acid (h2so4) will lose a proton to form its conjugate.
To Find The Conjugate Base Of An Acid, You Remove A Proton (H+) From It.
The conjugate base of h 2 s o 4 in the following reaction is: There are 2 steps to solve this one. The conjugate base of a substance is formed by removing a proton (h a +) from it. H 2 s o 4 + h 2 o → h 3 o + + h s o − 4
The Chemical Reaction For The Above.
The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak. Sulfuric acid (h2so4) will lose a proton to form its conjugate base.