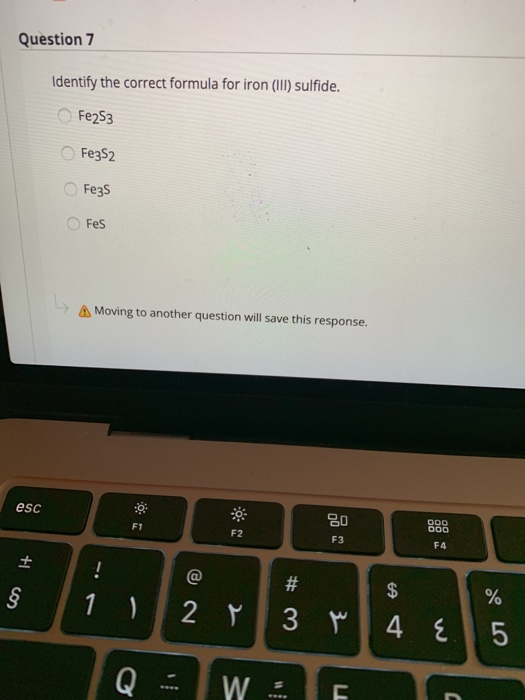
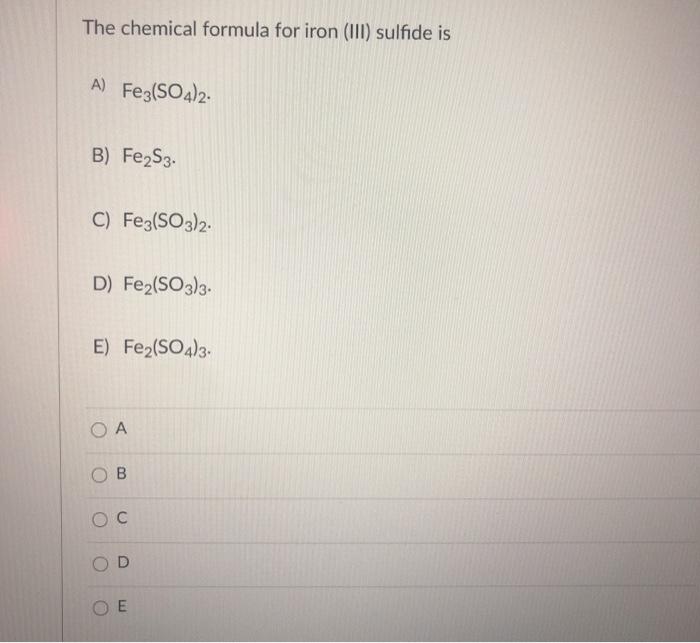
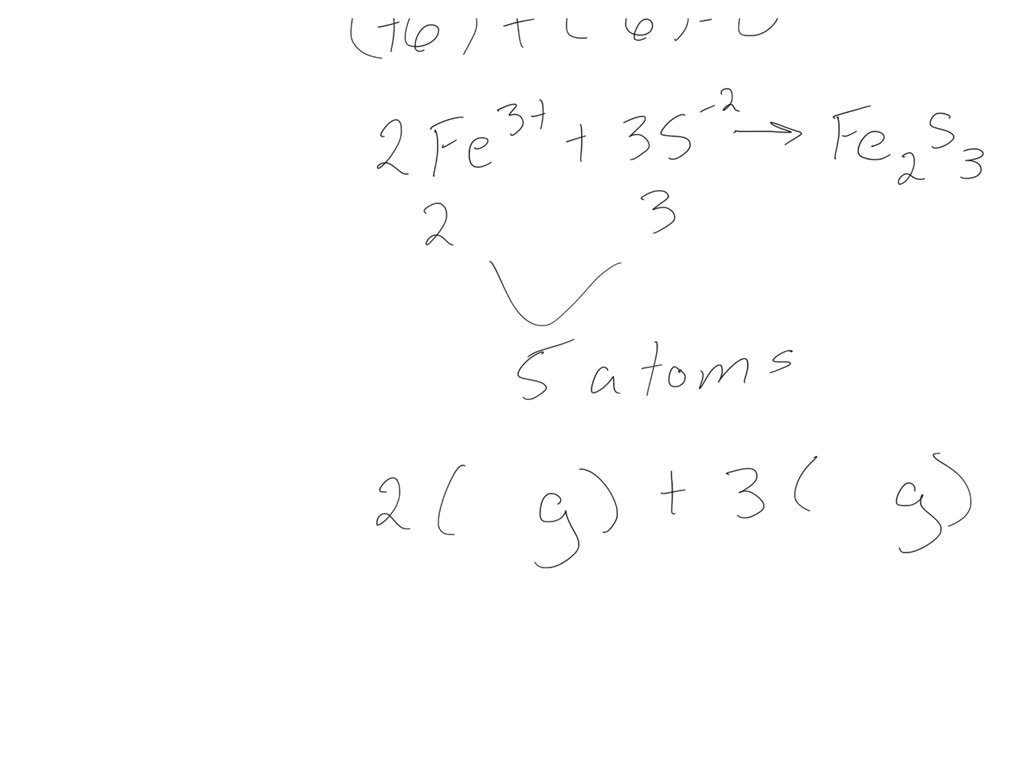What Is The Correct Formula For Iron Iii Sulfide - The charges have to add up to. Iron (fe) in iron(iii) has a +3 charge. To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. Formula for iron iii sulfide? The correct formula for iron(iii) sulfide is fe2s3. In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. In an ionic molecule the. The correct formula for iron(iii) sulfide is e) fe2s3. These are the ions and their charges: This is because iron(iii) indicates that iron has a charge of +3, and.
These are the ions and their charges: In an ionic molecule the. In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. The charges have to add up to. Formula for iron iii sulfide? The correct formula for iron(iii) sulfide is fe2s3. This is because iron(iii) indicates that iron has a charge of +3, and. Iron (fe) in iron(iii) has a +3 charge. The correct formula for iron(iii) sulfide is e) fe2s3. To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three.
In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. The correct formula for iron(iii) sulfide is e) fe2s3. This is because iron(iii) indicates that iron has a charge of +3, and. In an ionic molecule the. Formula for iron iii sulfide? The charges have to add up to. The correct formula for iron(iii) sulfide is fe2s3. To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. These are the ions and their charges: Iron (fe) in iron(iii) has a +3 charge.
Solved What is the correct chemical formula for Iron III Sulfide? a
In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. The correct formula for iron(iii) sulfide is fe2s3. In an ionic molecule the. These are the ions and their charges: The correct formula for iron(iii) sulfide is e) fe2s3.
Solved Question 7 Identify the correct formula for iron
In an ionic molecule the. The correct formula for iron(iii) sulfide is fe2s3. These are the ions and their charges: The charges have to add up to. The correct formula for iron(iii) sulfide is e) fe2s3.
(Get Answer) What Is The Correct Formula For Iron (II) Sulfide? O
In an ionic molecule the. The charges have to add up to. In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. This is because iron(iii) indicates that iron has a charge of +3, and. Formula for iron iii sulfide?
Solved The chemical formula for iron (III) sulfide is A)
Formula for iron iii sulfide? To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. Iron (fe) in iron(iii) has a +3 charge. This is because iron(iii) indicates that iron has a charge of +3, and. The charges have to add up to.
Iron(III) Sulfide Facts, Formula, Properties, Uses, Safety Data
In an ionic molecule the. These are the ions and their charges: This is because iron(iii) indicates that iron has a charge of +3, and. Formula for iron iii sulfide? Iron (fe) in iron(iii) has a +3 charge.
Solved A mixture containing only iron(III) sulfide and
The correct formula for iron(iii) sulfide is fe2s3. The correct formula for iron(iii) sulfide is e) fe2s3. These are the ions and their charges: In an ionic molecule the. This is because iron(iii) indicates that iron has a charge of +3, and.
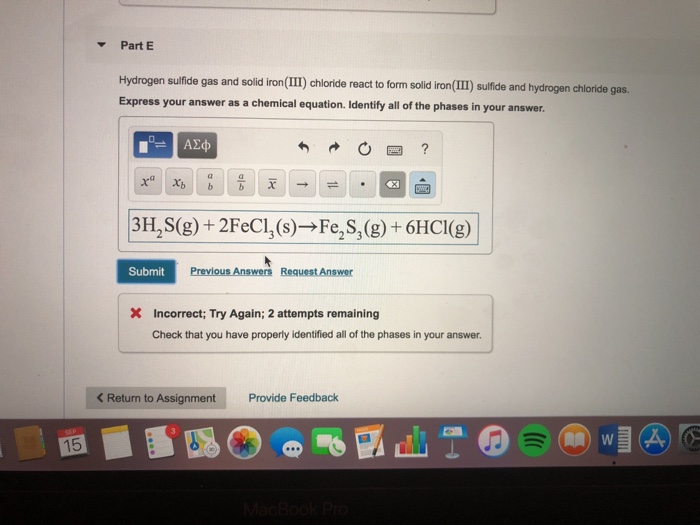
Solved Part E Hydrogen sulfide gas and solid iron (III)
Formula for iron iii sulfide? The correct formula for iron(iii) sulfide is e) fe2s3. To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. These are the ions and their charges: The correct formula for iron(iii) sulfide is fe2s3.
SOLVED For the compound iron (III) sulfide, answer the following
The correct formula for iron(iii) sulfide is fe2s3. In an ionic molecule the. This is because iron(iii) indicates that iron has a charge of +3, and. To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. The correct formula for iron(iii) sulfide is e) fe2s3.
Solved Compound Name Formula iron(III) sulfide nickel(II)
To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. The correct formula for iron(iii) sulfide is e) fe2s3. This is because iron(iii) indicates that iron has a charge of +3, and. Iron (fe) in iron(iii) has a +3 charge. In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and.
Solved Iron(III) sulfide reacts with HCl (g) to produce
The correct formula for iron(iii) sulfide is fe2s3. Formula for iron iii sulfide? To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. The charges have to add up to.
The Charges Have To Add Up To.
The correct formula for iron(iii) sulfide is fe2s3. Formula for iron iii sulfide? The correct formula for iron(iii) sulfide is e) fe2s3. In an ionic molecule the.
These Are The Ions And Their Charges:
In summary, the correct chemical formula for iron(iii) sulfide, based on oxidation states and charge balancing, is fe₂s₃. To balance the charges, we need two fe³⁺ ions (2 × +3 = +6) and three. This is because iron(iii) indicates that iron has a charge of +3, and. Iron (fe) in iron(iii) has a +3 charge.