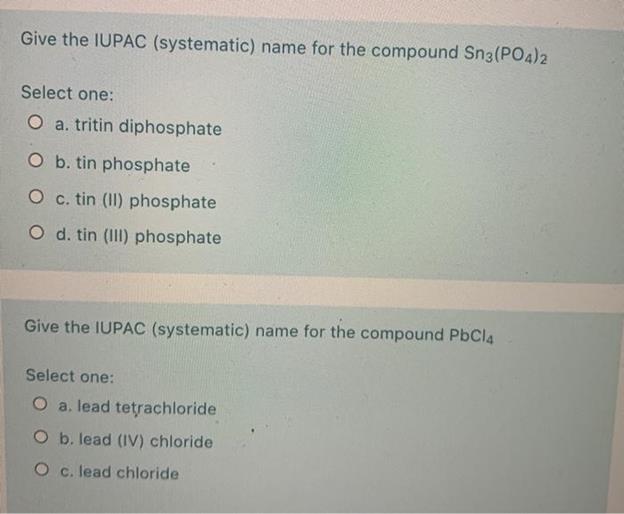
What Is The Correct Name For Sn3 Po4 2 - The trick here is to recognize the fact that you're dealing with a transition. Which set of chemical name and chemical formula for the same compound is correct? The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. What is the name for sn3(po4)2? In this compound, tin has a +2 oxidation state,. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. In this compound, tin acts as the cation with a +2.
The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. Which set of chemical name and chemical formula for the same compound is correct? The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. The trick here is to recognize the fact that you're dealing with a transition. In this compound, tin acts as the cation with a +2. What is the name for sn3(po4)2? The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. In this compound, tin has a +2 oxidation state,.
The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. The trick here is to recognize the fact that you're dealing with a transition. What is the name for sn3(po4)2? Which set of chemical name and chemical formula for the same compound is correct? In this compound, tin has a +2 oxidation state,. The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. In this compound, tin acts as the cation with a +2. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate.
Po4 Chemical Name And Structure Seller Vintage
The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. In this compound, tin has a +2 oxidation state,. The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. What is the name for sn3(po4)2? The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state.
Solved Write the name k) Sn3(PO4) l) NaBr m) Na2SO4 n)K3PO3
In this compound, tin has a +2 oxidation state,. The trick here is to recognize the fact that you're dealing with a transition. The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. In this compound, tin acts as the cation with a +2. Which set of chemical name and chemical formula for the same compound is correct?
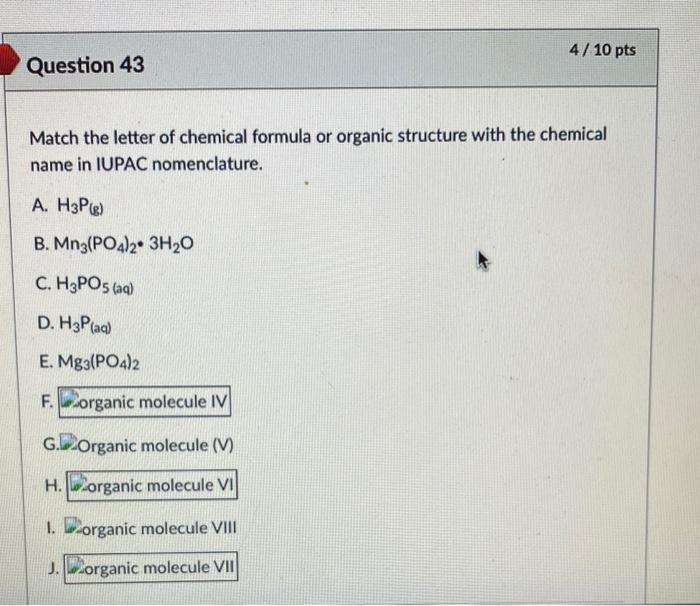
How to Write the Name for Sn3(PO4)2
The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. Which set of chemical name and chemical formula for the same compound is correct? The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. In this compound, tin acts as the cation.
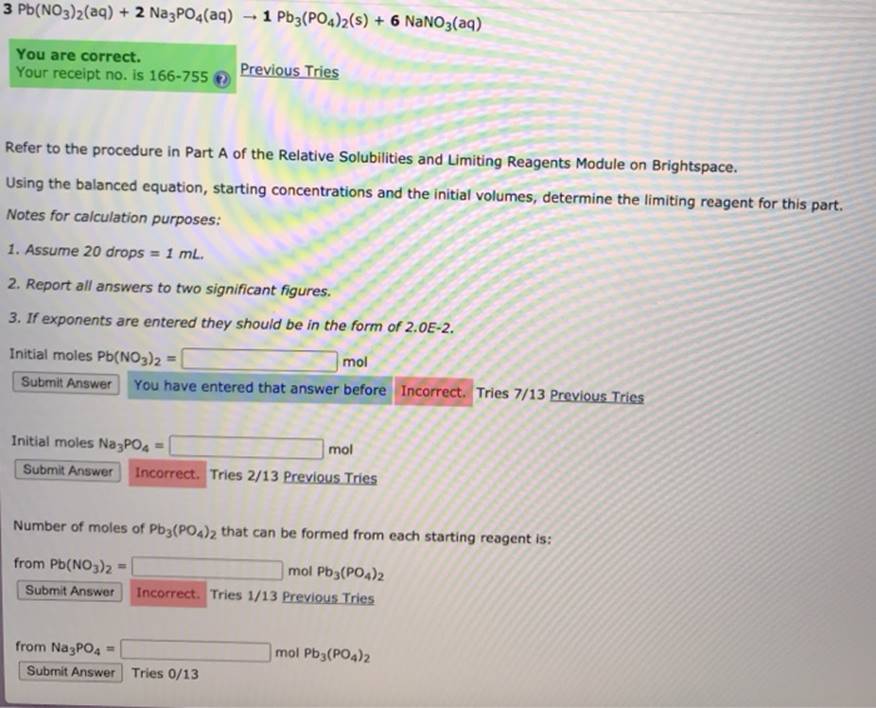
(Get Answer) 3 Pb(NO3)2(Aq) + 2 Na3PO4(Aq) 1 Pb3(PO4)2(S) + 6 NaNO3
The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. In this compound, tin has a +2 oxidation state,. The trick here is to recognize the fact that you're dealing with a.
SOLVEDThe names given for the following compounds are incorrect. Write
In this compound, tin has a +2 oxidation state,. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. In this compound, tin acts as the cation with a +2. What is the name for sn3(po4)2? The trick here is to recognize the fact that you're dealing with a transition.
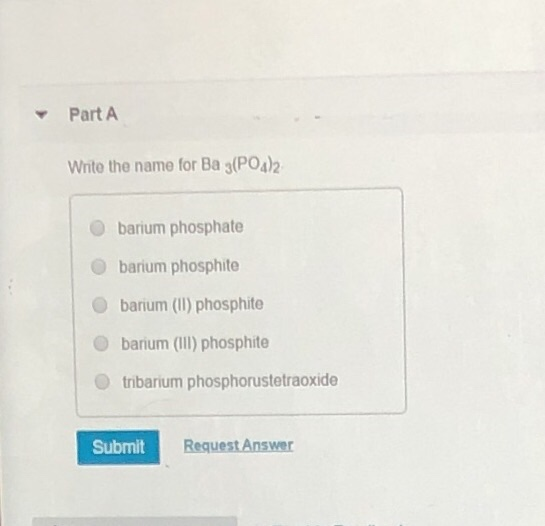
Solved Part A Write the name for Ba 3(PO4)2 barium phosphate
The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. The trick here is to recognize the fact that you're dealing with a transition. Which set of chemical name and chemical formula for the same compound is correct? In this compound, tin.
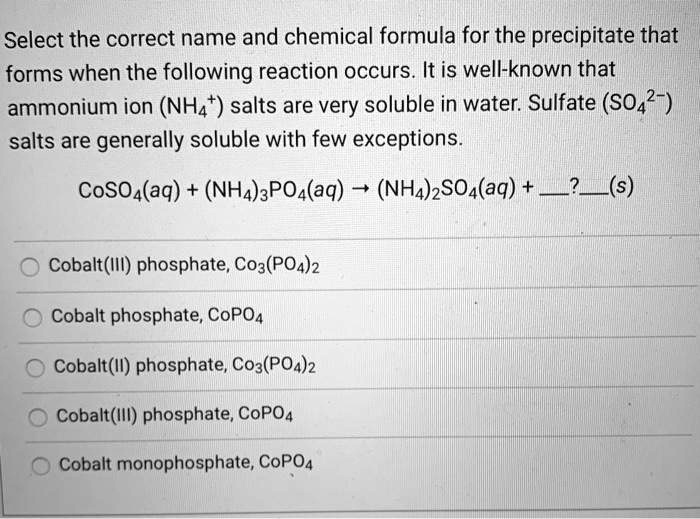
SOLVED Select the correct name and chemical formula for the
The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. In this compound, tin has a +2 oxidation state,. Which set of chemical name and chemical formula for the same compound is correct? In this compound, tin acts as the cation with a +2. The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation.
Po4 Chemical Name And Structure Seller Vintage
In this compound, tin has a +2 oxidation state,. The trick here is to recognize the fact that you're dealing with a transition. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. Which set of chemical name and chemical formula for the same compound is correct? The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'.
Solved 1Give the chemical formula for copper (II)
The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'. The correct name for the ionic compound sn3(po4)2 is tin(ii) phosphate. In this compound, tin acts as the cation with a +2. Which set of chemical name and chemical formula for the same compound is correct? What is the name for sn3(po4)2?
(Get Answer) Transcribed image text Give the IUPAC (systematic
What is the name for sn3(po4)2? The trick here is to recognize the fact that you're dealing with a transition. In this compound, tin acts as the cation with a +2. In this compound, tin has a +2 oxidation state,. The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'.
The Correct Name For The Ionic Compound Sn3(Po4)2 Is Tin(Ii) Phosphate.
Which set of chemical name and chemical formula for the same compound is correct? What is the name for sn3(po4)2? The correct name for sn₃(po₄)₂ is tin(iv) phosphate, indicating that tin is in the +4 oxidation state. The correct name for the compound sn₃(po₄)₂ is 'tin(ii) phosphate'.
In This Compound, Tin Acts As The Cation With A +2.
In this compound, tin has a +2 oxidation state,. The trick here is to recognize the fact that you're dealing with a transition.