What Is The Density Of Carbon Dioxide At Stp - The density of carbon dioxide at stp is approximately 1.95 g/l. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. 1 mol occupies 22400 cm3. To calculate the density of carbon dioxide at stp, we use the. At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e. The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: So, the density of co2 at s.t.p is. One mole of an ideal gas at standard temperature and pressure has.
The density of carbon dioxide at stp is 1.964 grams per liter (g/l). The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. To calculate the density of carbon dioxide at stp, we use the. Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. 1 mol occupies 22400 cm3. So, the density of co2 at s.t.p is. The density of carbon dioxide at stp is approximately 1.95 g/l. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: One mole of an ideal gas at standard temperature and pressure has. At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e.
At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. To calculate the density of carbon dioxide at stp, we use the. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: One mole of an ideal gas at standard temperature and pressure has. So, the density of co2 at s.t.p is. Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. The density of carbon dioxide at stp is approximately 1.95 g/l. 1 mol occupies 22400 cm3.
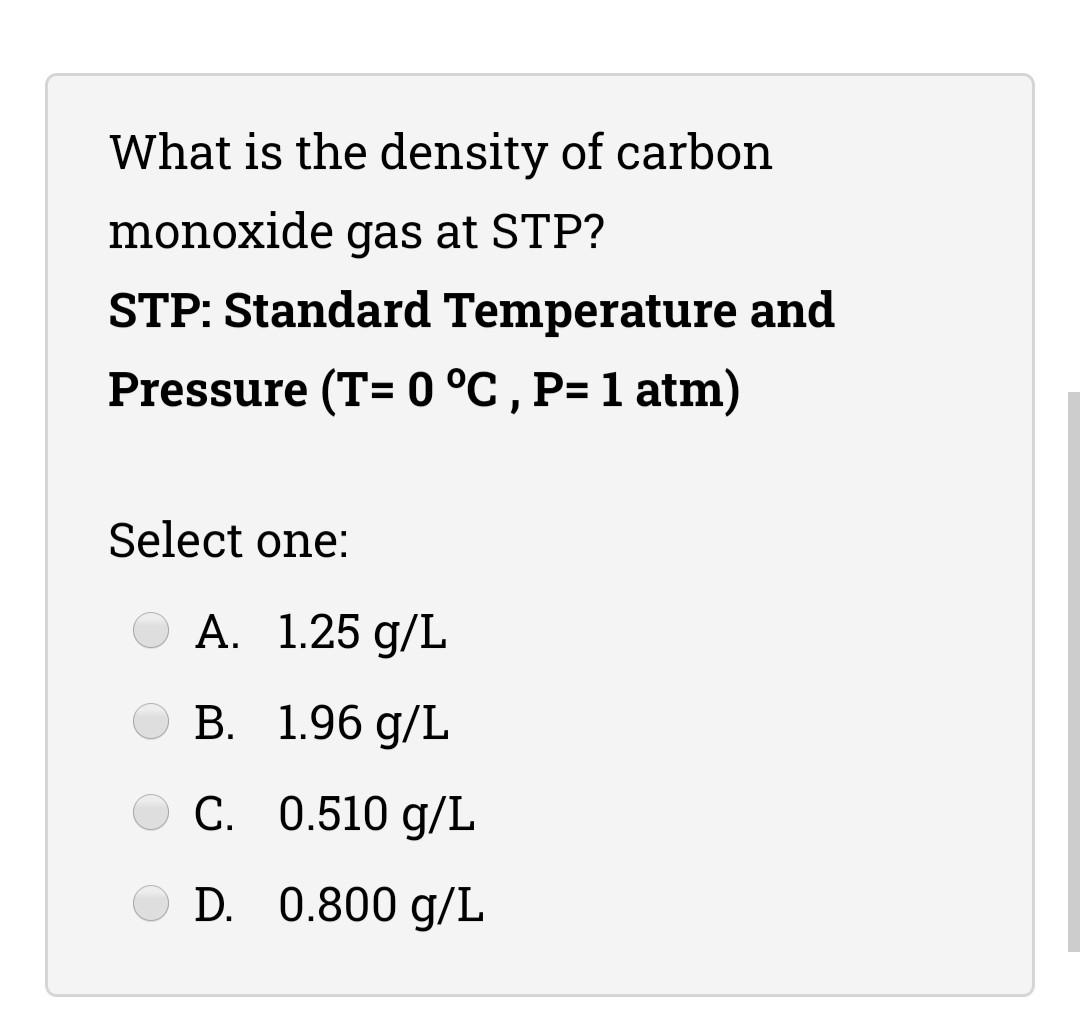
Solved What is the density of carbon monoxide gas at STP?
At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. To calculate.
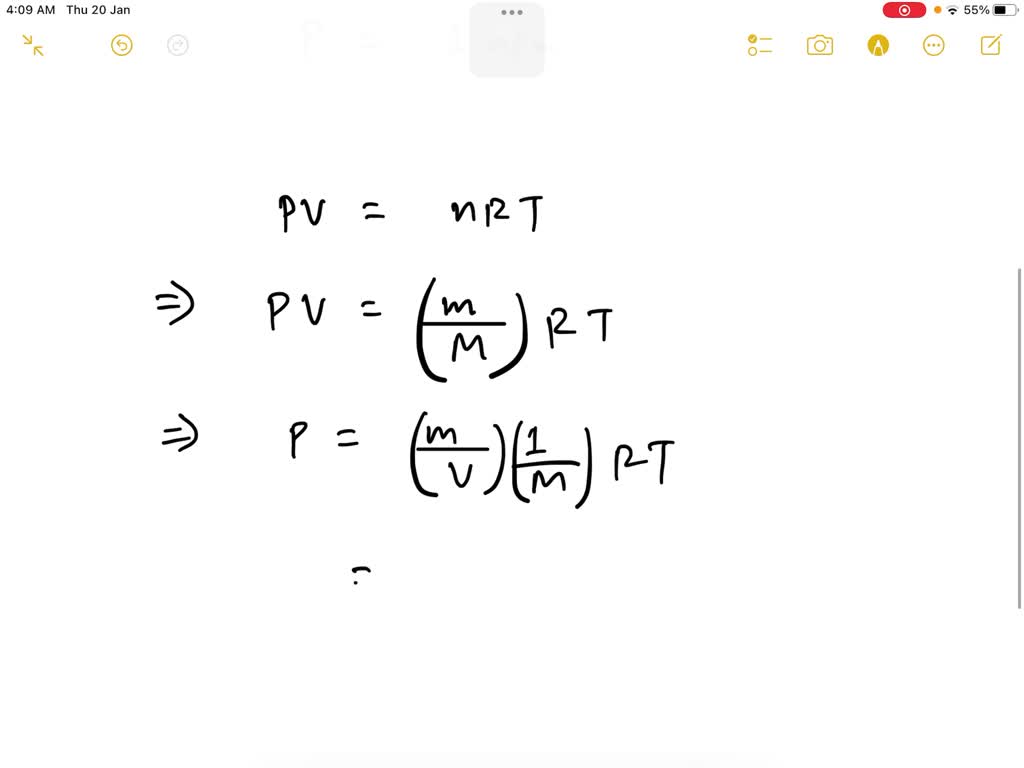
SOLVED Use the molar volume of a gas at STP to calculate the density
The density of carbon dioxide at stp is approximately 1.95 g/l. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. To calculate the density of carbon dioxide at stp, we.
SOLVED Which of the following gases has the lowest density at STP? a
Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. 1 mol occupies 22400 cm3. So, the density of co2 at s.t.p is. The density of carbon dioxide at stp is approximately 1.95 g/l. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps:
Density of Carbon Dioxide khawkins17
To calculate the density of carbon dioxide at stp, we use the. 1 mol occupies 22400 cm3. The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). One mole of an.
SOLVED Assuming it behaves as an ideal gas, calculate the density of
The density of carbon dioxide at stp is 1.964 grams per liter (g/l). The density of carbon dioxide at stp is approximately 1.95 g/l. So, the density of co2 at s.t.p is. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: At s.t.p, the molar mass of co2 occupies 22.4 l.
The volume occupied by 80 g of carbon dioxide STP.
So, the density of co2 at s.t.p is. 1 mol occupies 22400 cm3. The density of carbon dioxide at stp is approximately 1.95 g/l. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps:
What Is the Density of Carbon Dioxide at Stp
Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). At s.t.p, the molar mass of.
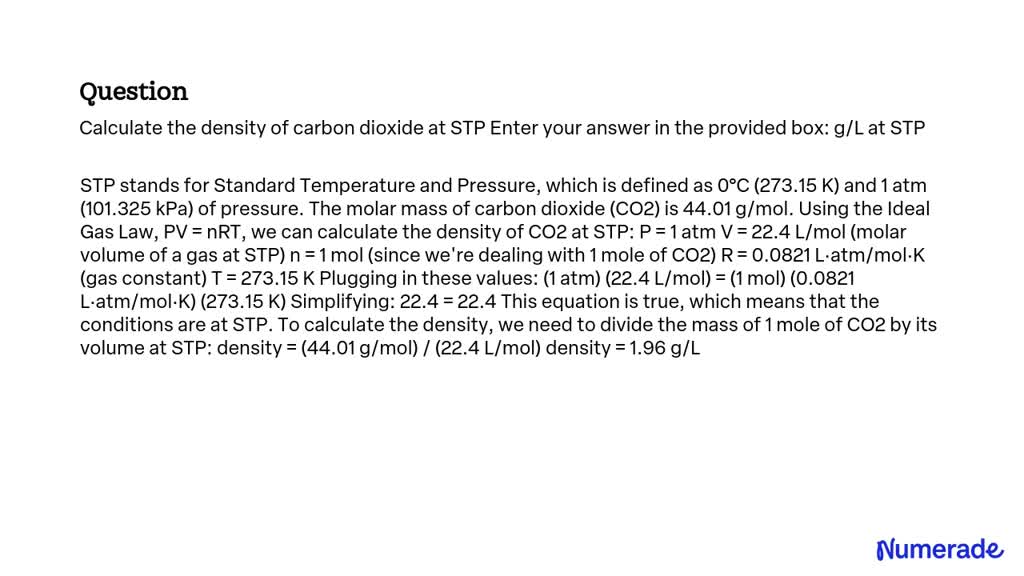
SOLVED Calculate the density of carbon dioxide at STP Enter your
Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e. 1 mol occupies 22400 cm3. The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon..
Density profiles vs. temperature for carbon dioxide, helium, water and
1 mol occupies 22400 cm3. To calculate the density of carbon dioxide at stp, we use the. One mole of an ideal gas at standard temperature and pressure has. The density of carbon dioxide at stp is 1.964 grams per liter (g/l). The density of carbon dioxide at stp is approximately 1.95 g/l.
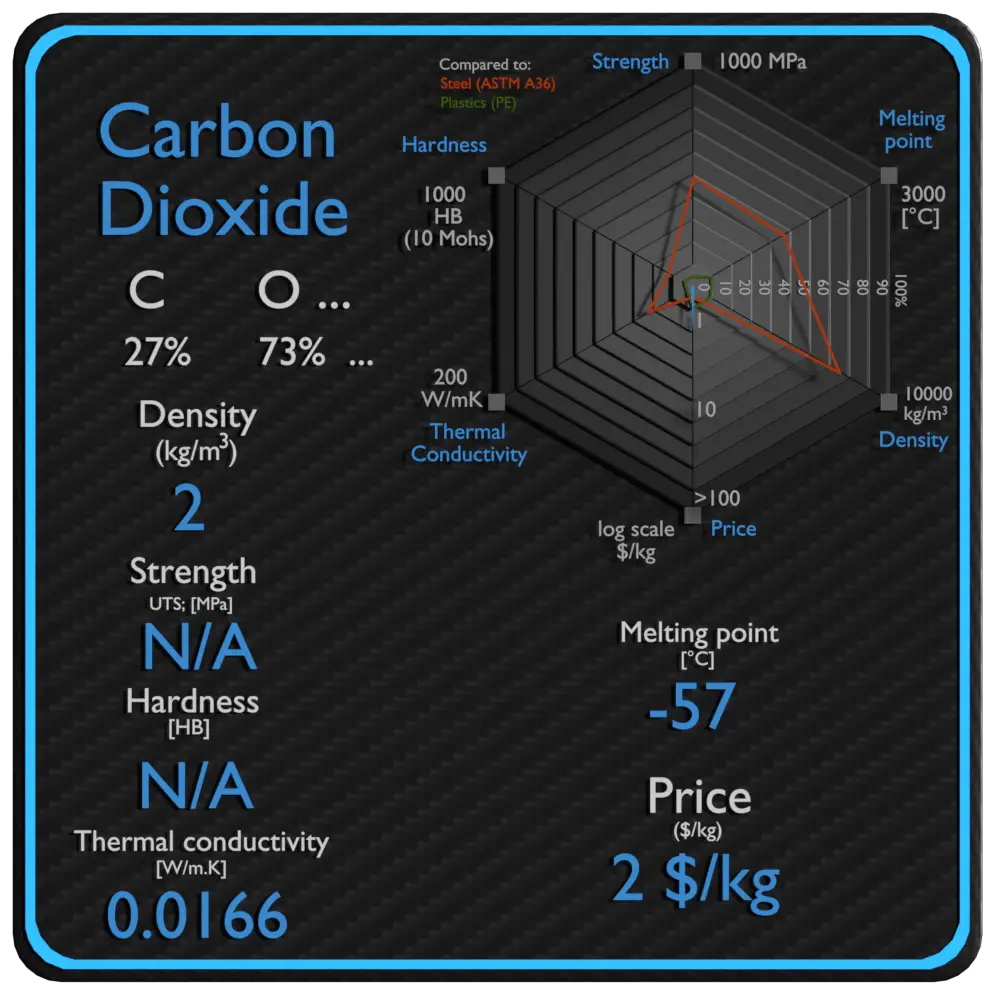
Carbon Dioxide Density, Heat Capacity, Thermal Conductivity
The density of carbon dioxide at stp is approximately 1.95 g/l. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e. Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. So, the.
1 Mol Occupies 22400 Cm3.
So, the density of co2 at s.t.p is. At s.t.p, the molar mass of co2 occupies 22.4 l = 22400 cm3, i.e. To find the density of carbon dioxide (co2) gas at standard temperature and pressure (stp), follow these steps: The density of carbon dioxide at stp is 1.964 grams per liter (g/l).
To Calculate The Density Of Carbon Dioxide At Stp, We Use The.
Densities and molecular weights of common gases like acetylene, air, methane, nitrogen, oxygen and others. The density of carbon dioxide at stp is 1.964 g/l explanation 1 the molar mass of carbon dioxide (co2) is 44.01 g/mol (12.01 g/mol for carbon. The density of carbon dioxide at stp is approximately 1.95 g/l. One mole of an ideal gas at standard temperature and pressure has.