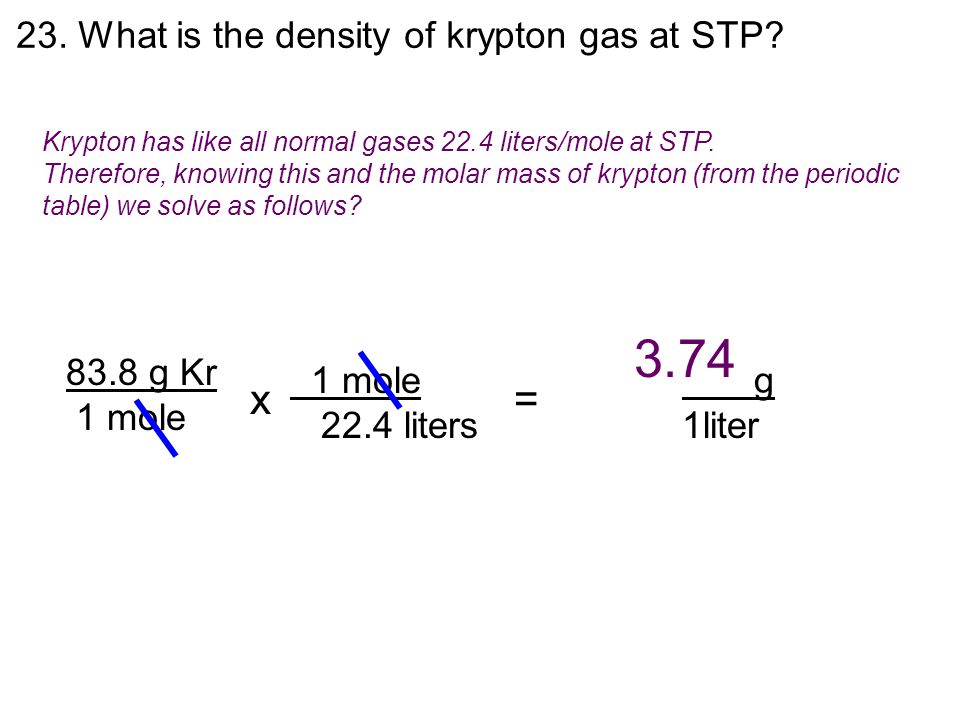
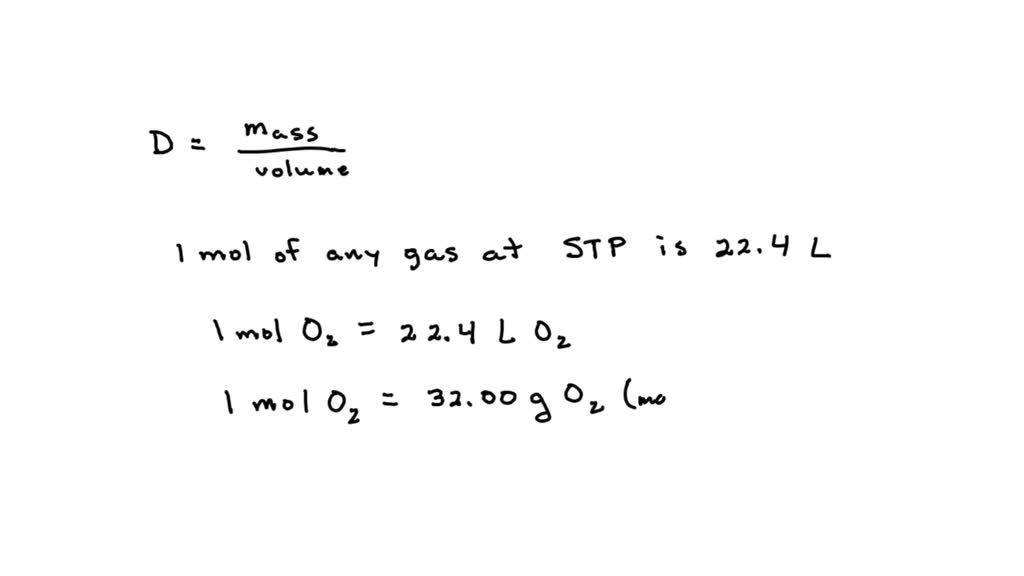What Is The Density Of Krypton Gas At Stp - Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. Pv = nrt, where p is pressure, v is volume, n is the. The density of a gas at stp can be calculated using the ideal gas law: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The equation used to calculate the density is: How do you find the density of krypton?
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. How do you find the density of krypton? The equation used to calculate the density is: Pv = nrt, where p is pressure, v is volume, n is the. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The density of a gas at stp can be calculated using the ideal gas law:
The density of a gas at stp can be calculated using the ideal gas law: Pv = nrt, where p is pressure, v is volume, n is the. How do you find the density of krypton? The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The equation used to calculate the density is:
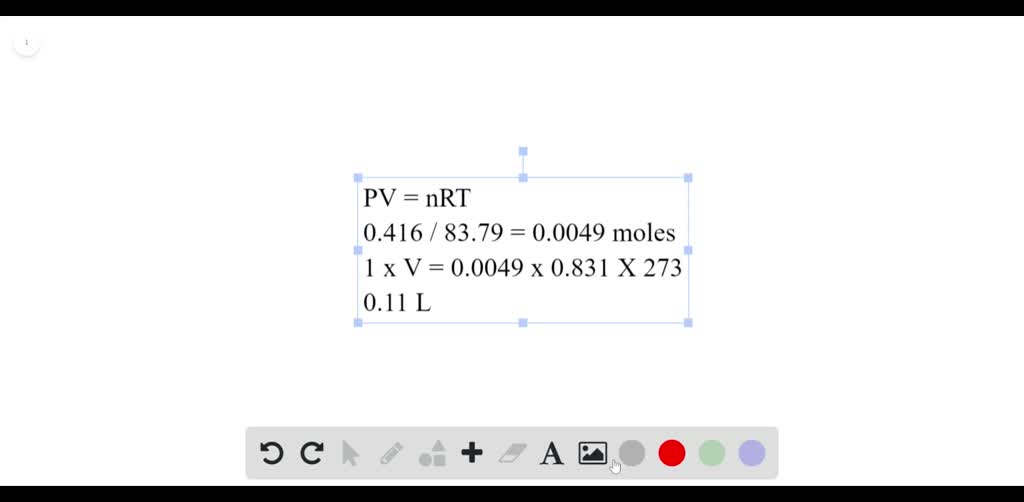
What volume will 0.416 g of krypton gas occupy at STP? Numerade
How do you find the density of krypton? Pv = nrt, where p is pressure, v is volume, n is the. Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The equation used to calculate the density is: The density of an ideal gas at standard temperature and pressure (stp), which is defined as.
Solved A sample of Krypton gas at STP occupies 40.1 liters.
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of a gas at stp can be calculated using the ideal gas law: The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. How do you find the density of krypton? Pv =.
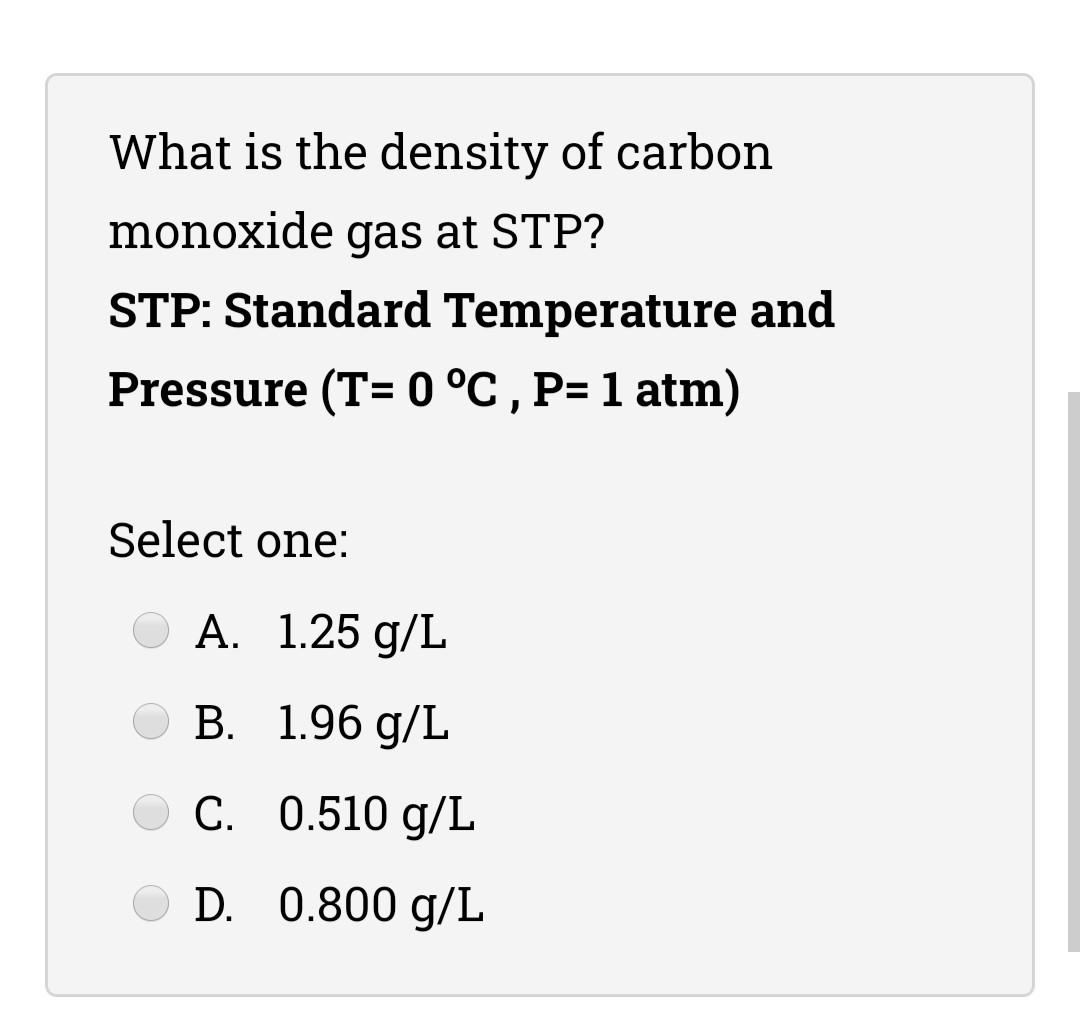
(Solved) What Is The Density Of Carbon Monoxide Gas At STP? STP
Pv = nrt, where p is pressure, v is volume, n is the. Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of a gas at stp can be calculated using the ideal gas law: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc.
What Is the Density of Krypton Gas at Stp
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. Pv = nrt, where p is pressure, v is volume, n is the. The equation used to calculate the density is: How do you find the density of krypton? The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of.
What Is the Density of Krypton Gas at Stp
The density of a gas at stp can be calculated using the ideal gas law: Pv = nrt, where p is pressure, v is volume, n is the. The equation used to calculate the density is: How do you find the density of krypton? The density of an ideal gas at standard temperature and pressure (stp), which is defined as.
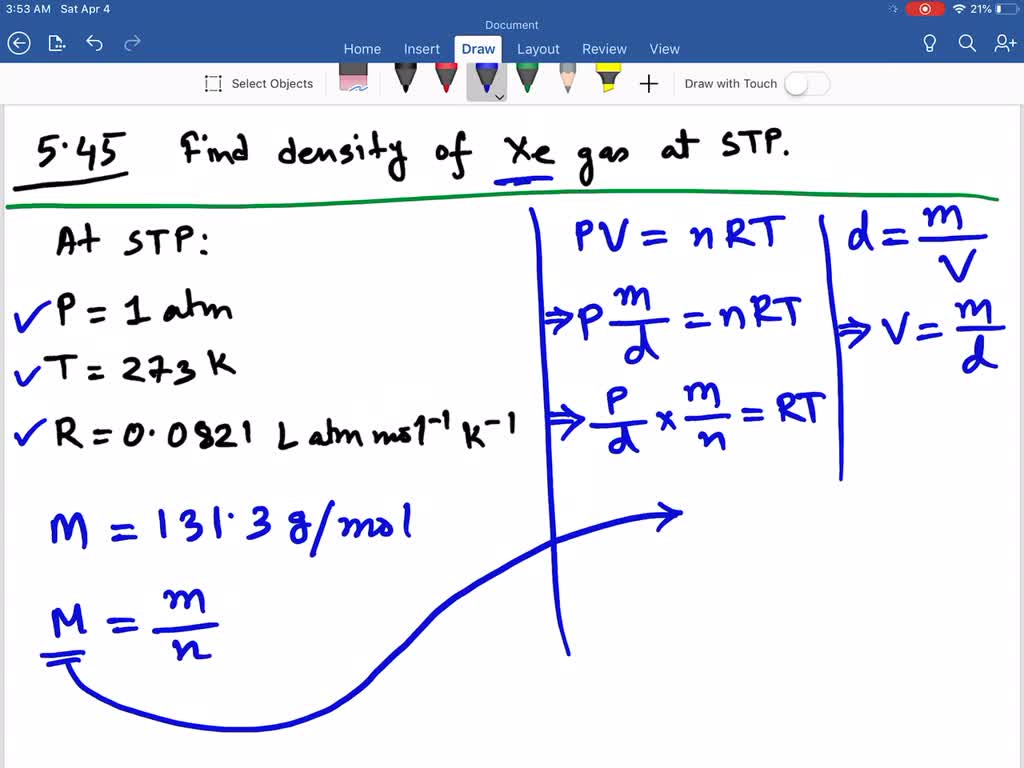
SOLVEDWhat is the density of Xe gas at STP?
The density of a gas at stp can be calculated using the ideal gas law: Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. The equation used to calculate the density is: How do.
Calculate the density of oxygen gas at STP using the molar volume of
Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. How do you find the density of krypton? The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc.
27. The density of a gas STP is 1.5g/L STP. Its molecular weight is 1
Pv = nrt, where p is pressure, v is volume, n is the. How do you find the density of krypton? Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density of a gas at stp can be calculated using the ideal gas law: The equation used to calculate the density is:
What Is the Density of Krypton Gas at Stp
Pv = nrt, where p is pressure, v is volume, n is the. The equation used to calculate the density is: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The density of a gas at stp can be calculated using the ideal gas law: The density of.
What Is the Density of Krypton Gas at Stp
The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. The density of a gas at stp can be calculated using the ideal gas law: How do you find the density of krypton? Density = mass / volume mass of krypton = 83.798 g the volume occupied any. The density.
Pv = Nrt, Where P Is Pressure, V Is Volume, N Is The.
The density of a gas at stp can be calculated using the ideal gas law: The density of krypton at stp is 3.74 kg/m3 , or 0.00374 g/cm3 at stp of 0oc (273.15 k) and 1. The density of an ideal gas at standard temperature and pressure (stp), which is defined as a temperature of 0°c. How do you find the density of krypton?
The Equation Used To Calculate The Density Is:
Density = mass / volume mass of krypton = 83.798 g the volume occupied any.