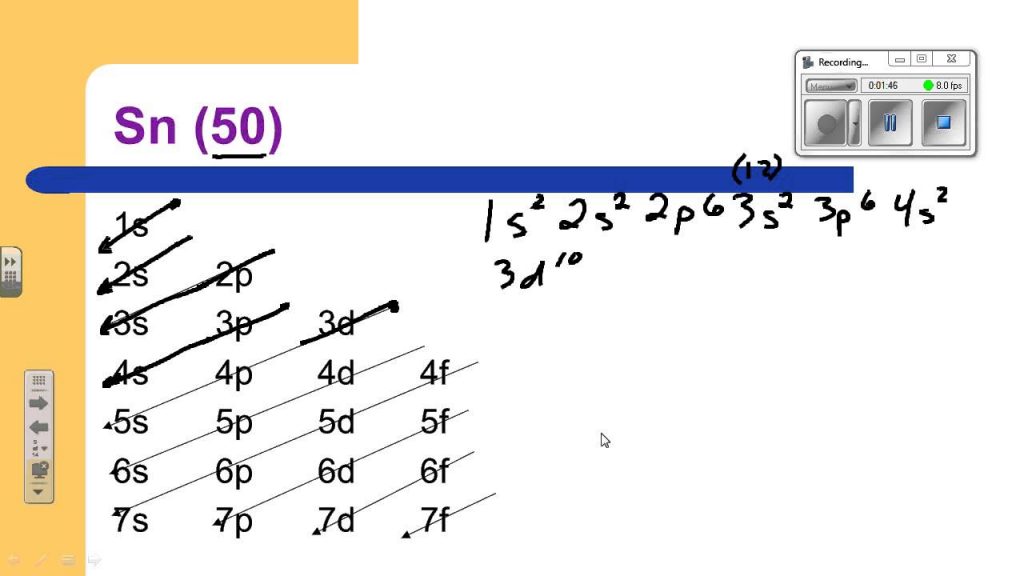
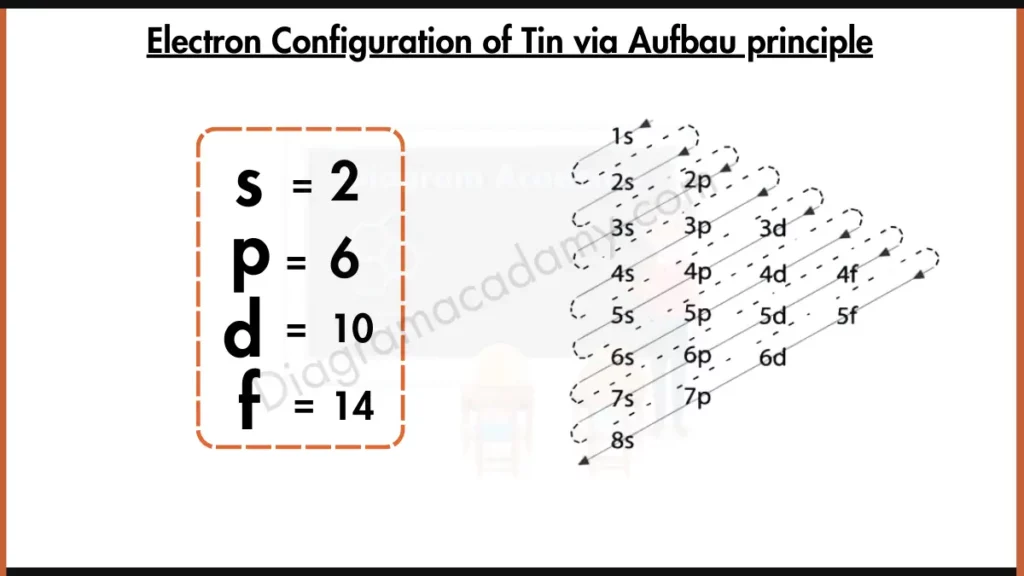
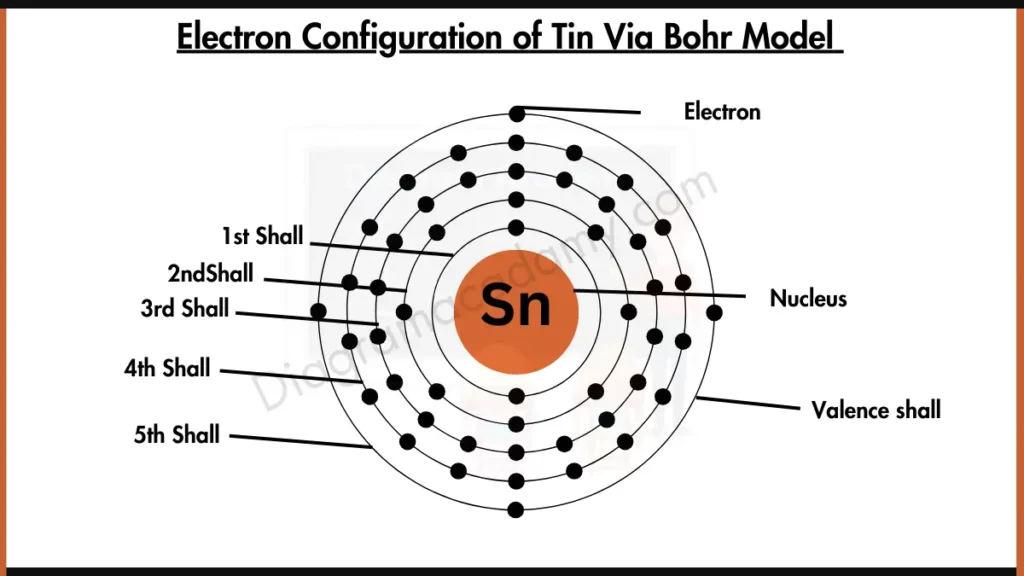
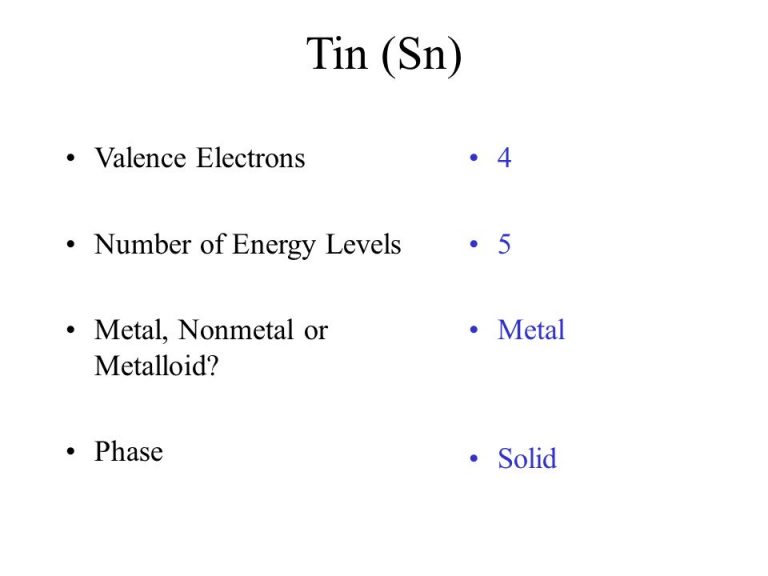
What Is The Electron Configuration For Tin - Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is. Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and.
Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and. Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.
Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is. Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and.
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+)
Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and. Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+)
Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is. Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and.
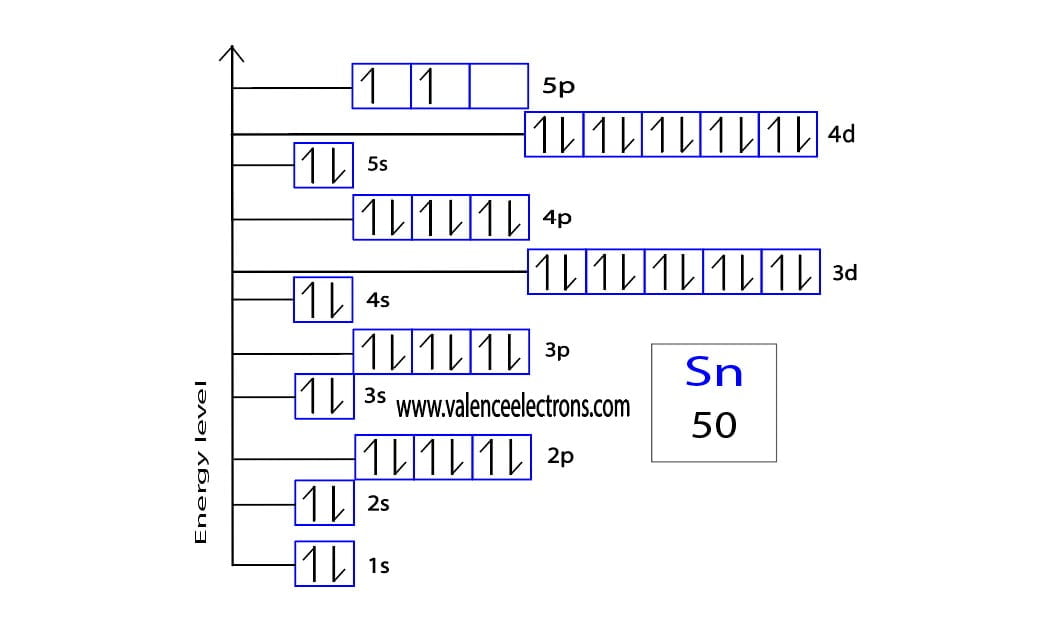
Tin Electron Configuration (Sn) with Orbital Diagram
Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and. Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+)
Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is. Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and.
Tin Electron Configuration (Sn) with Orbital Diagram
Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and. Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.
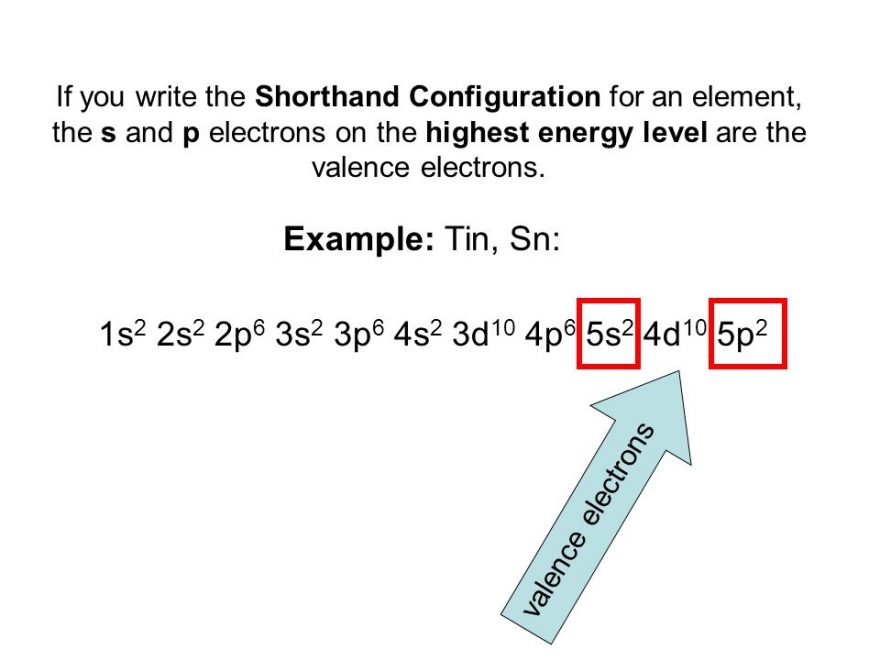
Electron Configuration of Tin Diagram
Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and. Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.
Tin(Sn) electron configuration and orbital diagram
Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is. Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and.
Electron Configuration of Tin Diagram
Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is. Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and.
Tin Electron Configuration (Sn) with Orbital Diagram
Tin has a ground state electron configuration of 1s22s22p63s23p64s23d104p65s24d105p2 and. Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.
Tin Has A Ground State Electron Configuration Of 1S22S22P63S23P64S23D104P65S24D105P2 And.
Tin is a chemical element of the periodic table with chemical symbol sn and atomic number 50 with an atomic weight of 118.711 u and is.