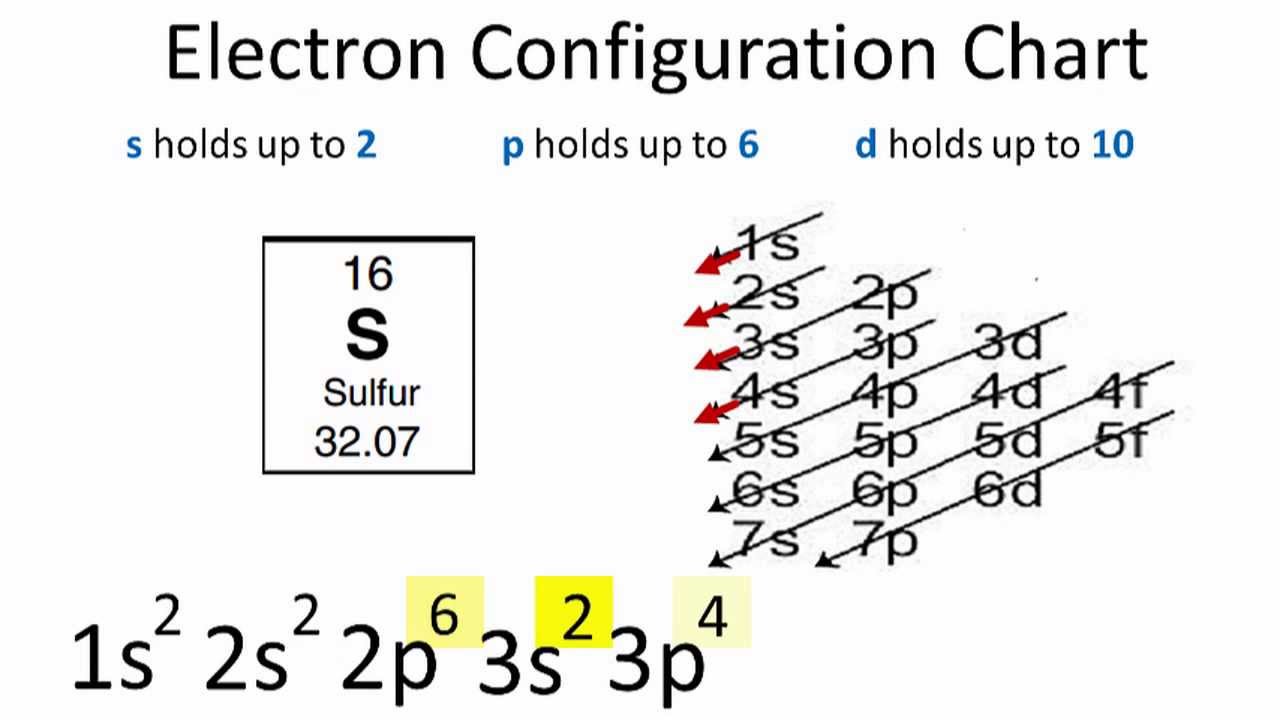
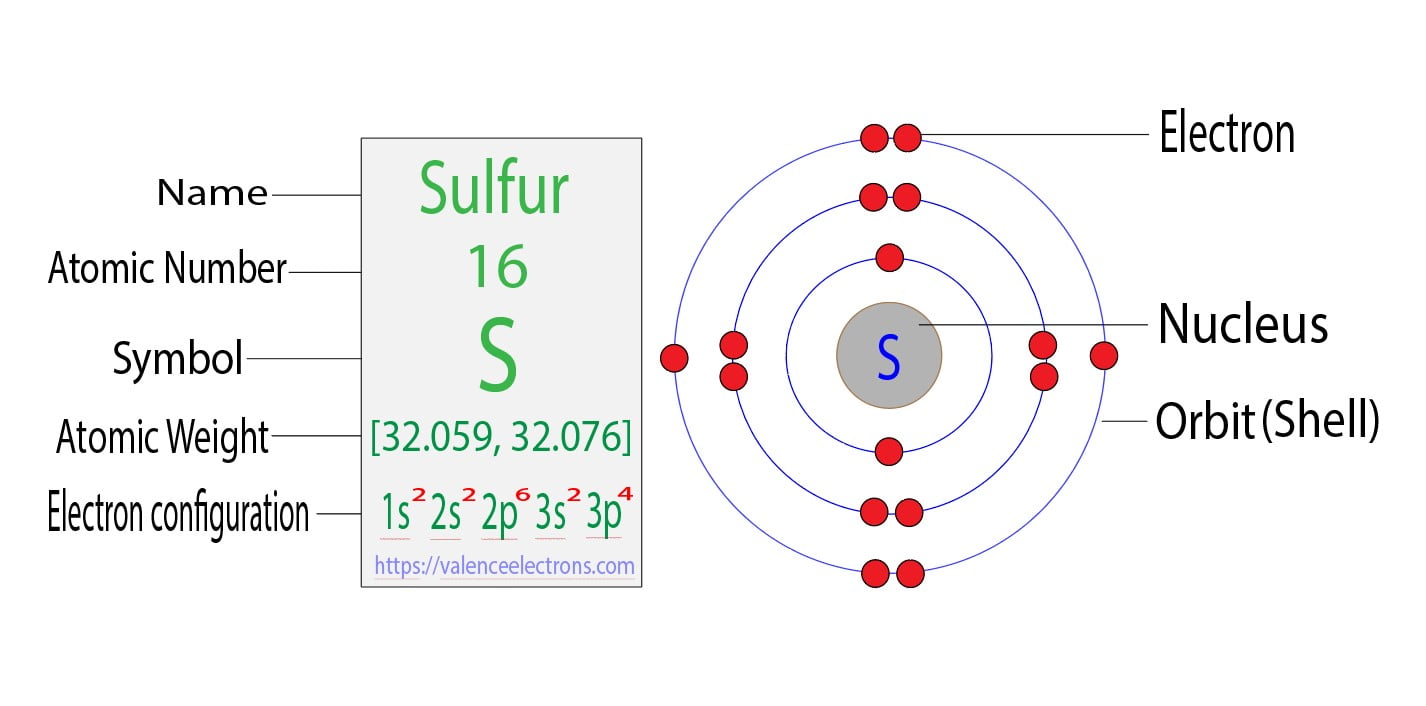
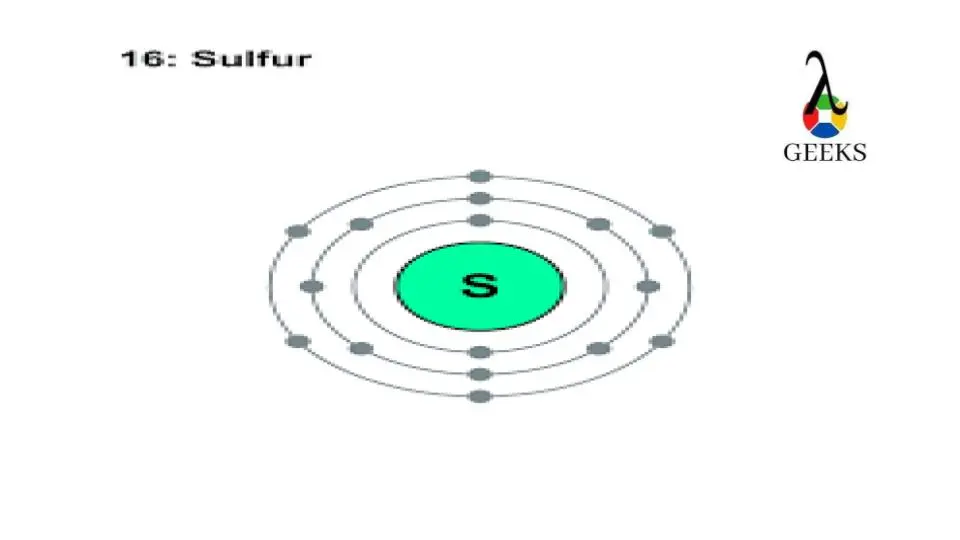
What Is The Electron Configuration Of Sulfur - In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. To understand the electron configuration of sulfur, we need to know its. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. This configuration follows the aufbau principle,.
The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). To understand the electron configuration of sulfur, we need to know its. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. This configuration follows the aufbau principle,.
The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). This configuration follows the aufbau principle,. To understand the electron configuration of sulfur, we need to know its. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4.
Sulfur Electron Configuration Jacks Of Science
To understand the electron configuration of sulfur, we need to know its. This configuration follows the aufbau principle,. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons)..
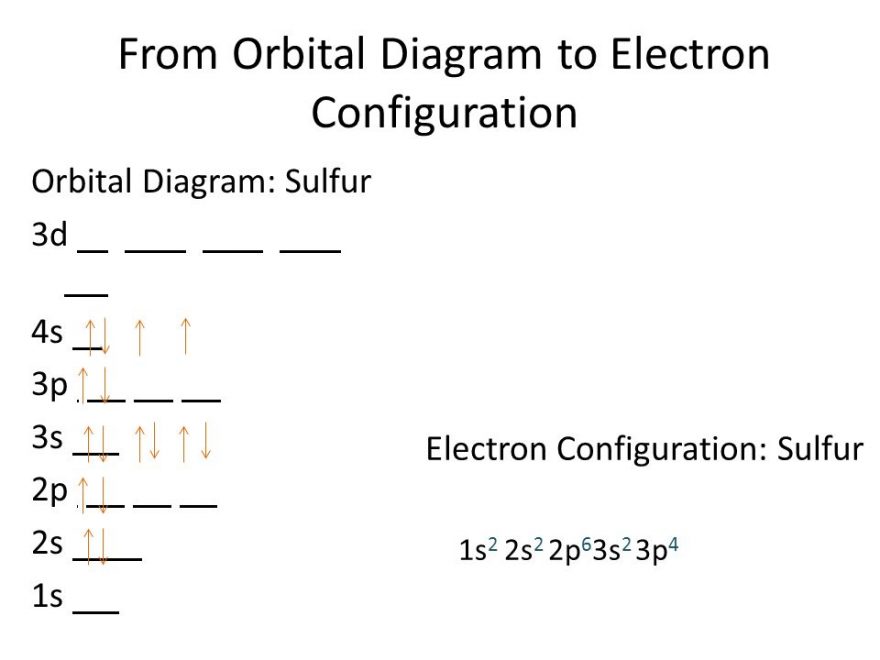
Sulfur Electron Configuration (S) with Orbital Diagram
Electron configuration chart of all elements is mentioned in the table below. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). The shorthand electron configuration (or noble gas. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. The electron configuration for.
Sulfur Electron Configuration (S) with Orbital Diagram
In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). This configuration follows the aufbau principle,. The shorthand electron configuration (or noble gas. To understand the electron configuration of sulfur, we need to know its. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6.
How to Write the Electron Configuration for Sulfur (S)?
The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. The shorthand electron configuration (or noble gas. To understand the electron configuration of sulfur, we need to know its. This configuration follows the aufbau principle,. In order to write the sulfur electron configuration we first need to know the number of electrons for.
Sulfur Electron Configuration5 Easy StepbyStep Guide!
Electron configuration chart of all elements is mentioned in the table below. To understand the electron configuration of sulfur, we need to know its. The shorthand electron configuration (or noble gas. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4.
Sulfur Electron Configuration Clipart (2243341) PinClipart
The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. Electron configuration chart of all elements is mentioned in the table below. This configuration follows the aufbau principle,. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons)..
Sulfur Electron Configuration Jacks Of Science
The shorthand electron configuration (or noble gas. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. To understand the electron configuration of sulfur, we need to know its. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. This configuration follows the aufbau principle,.
Sulfur Electron Configuration Jacks Of Science
In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). To understand the electron configuration of sulfur, we need to know its. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. The electron configuration for sulfur, which has 16 electrons, is represented.
Sulfur Electron Configuration Jacks Of Science
In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). This configuration follows the aufbau principle,. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴..
Sulfur Electron Configuration Jacks Of Science
To understand the electron configuration of sulfur, we need to know its. The electron configuration for sulfur, which has 16 electrons, is represented as 1s² 2s² 2p⁶ 3s² 3p⁴. The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas.
This Configuration Follows The Aufbau Principle,.
Electron configuration chart of all elements is mentioned in the table below. To understand the electron configuration of sulfur, we need to know its. The shorthand electron configuration (or noble gas. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons).
The Electron Configuration For Sulfur, Which Has 16 Electrons, Is Represented As 1S² 2S² 2P⁶ 3S² 3P⁴.
The electron configuration for sulfur (s) is 1s^2 2s^2 2p^6 3s^2 3p^4.