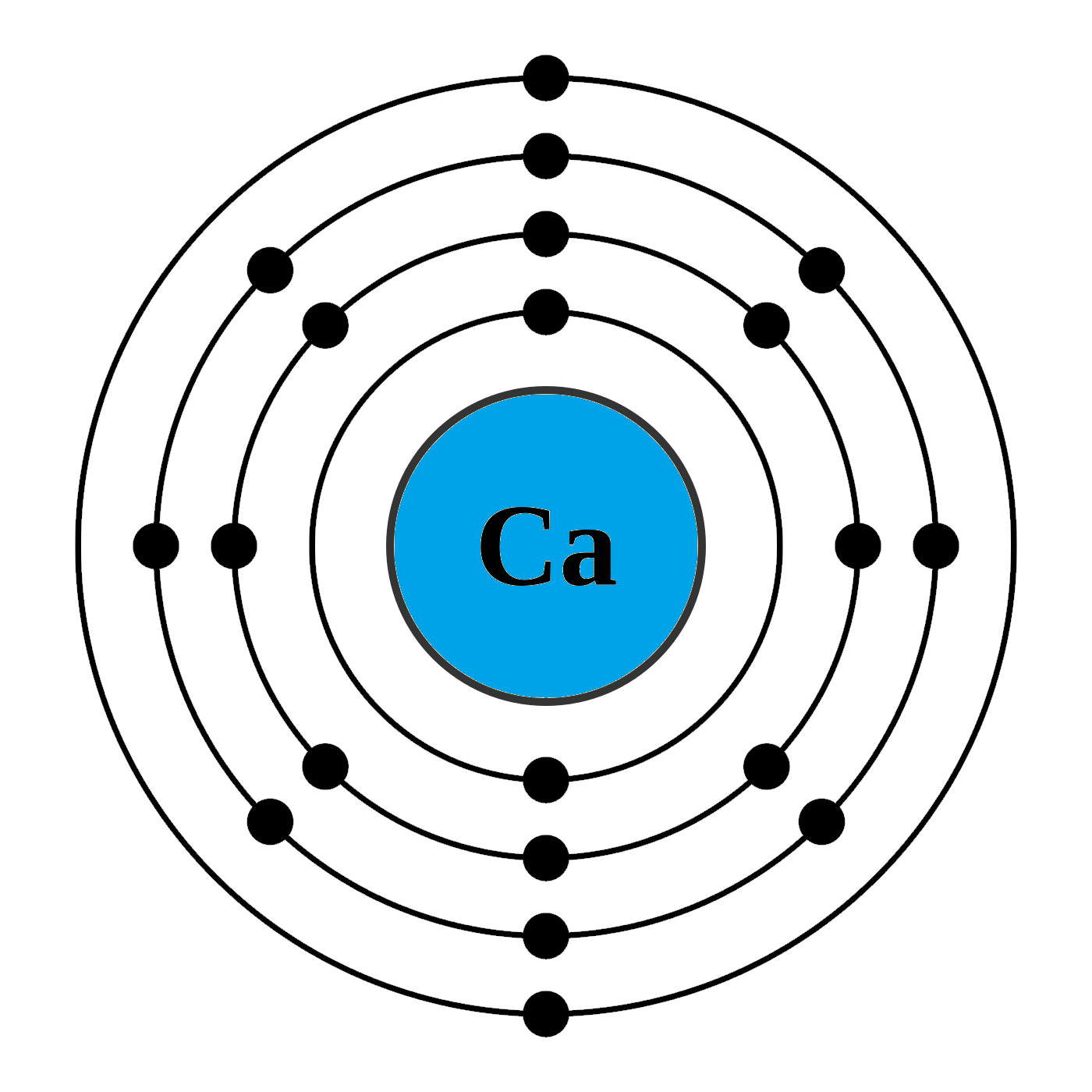
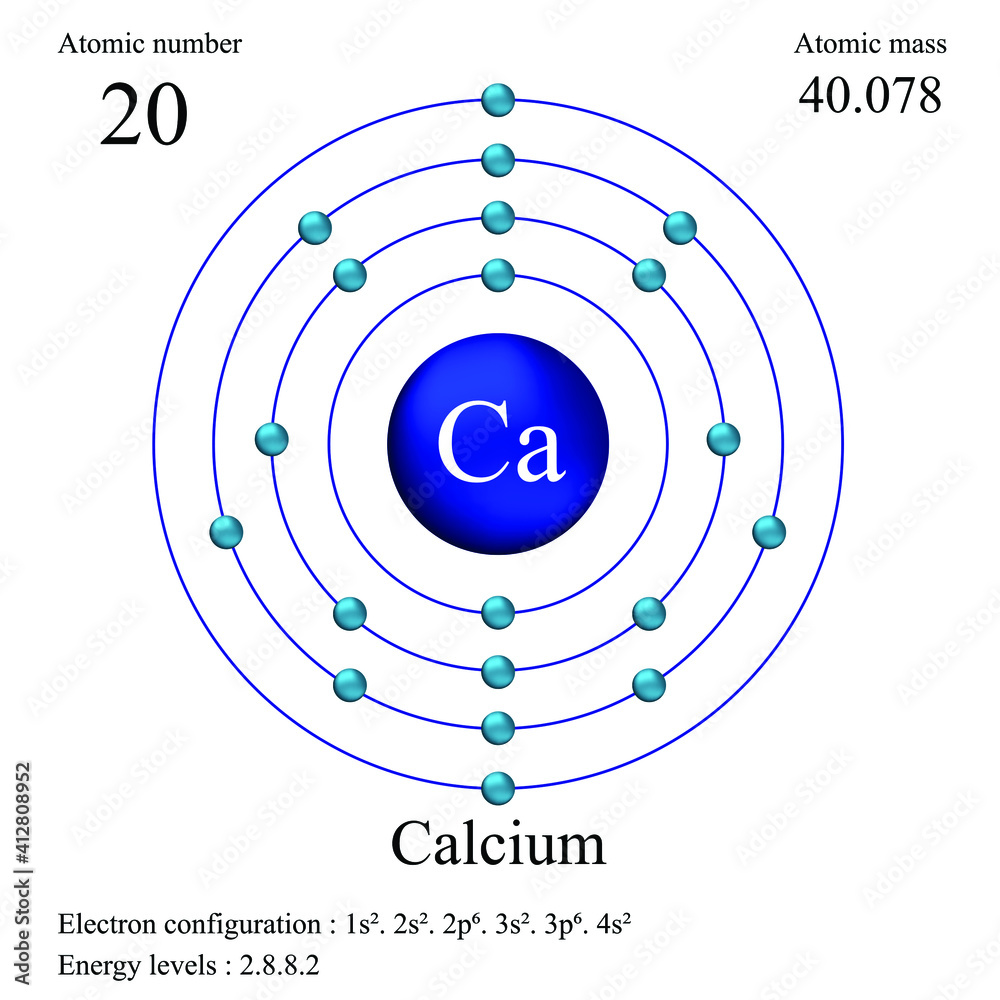
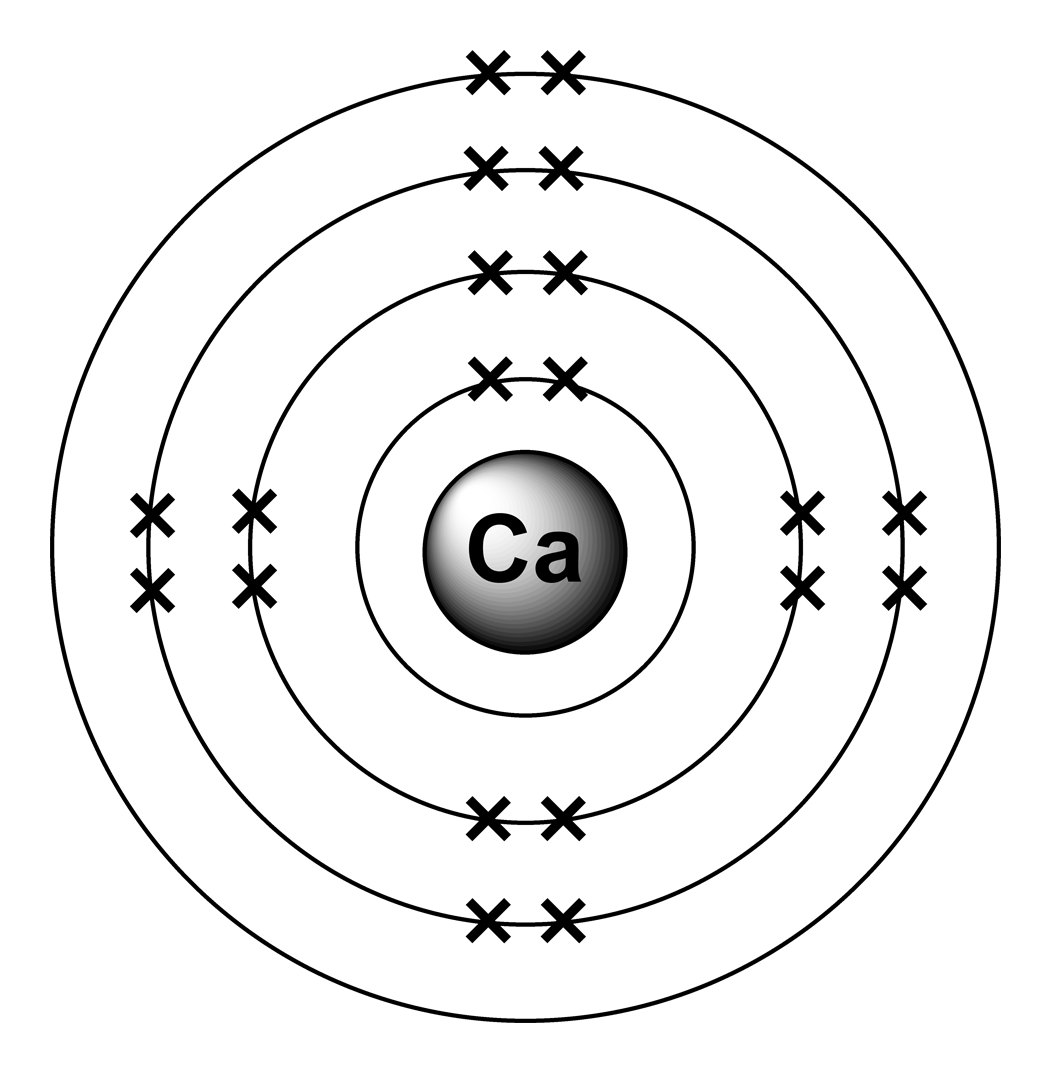
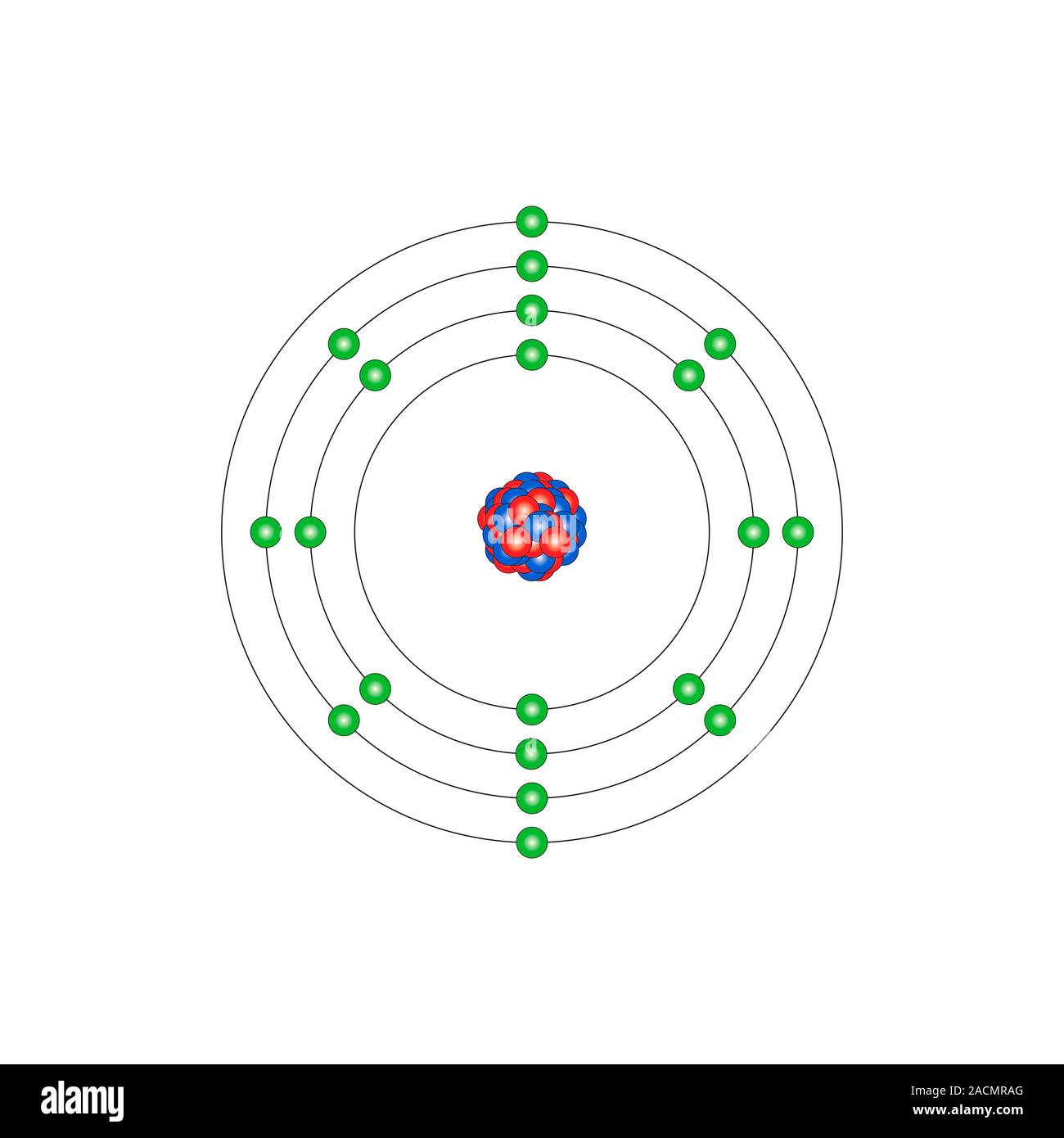
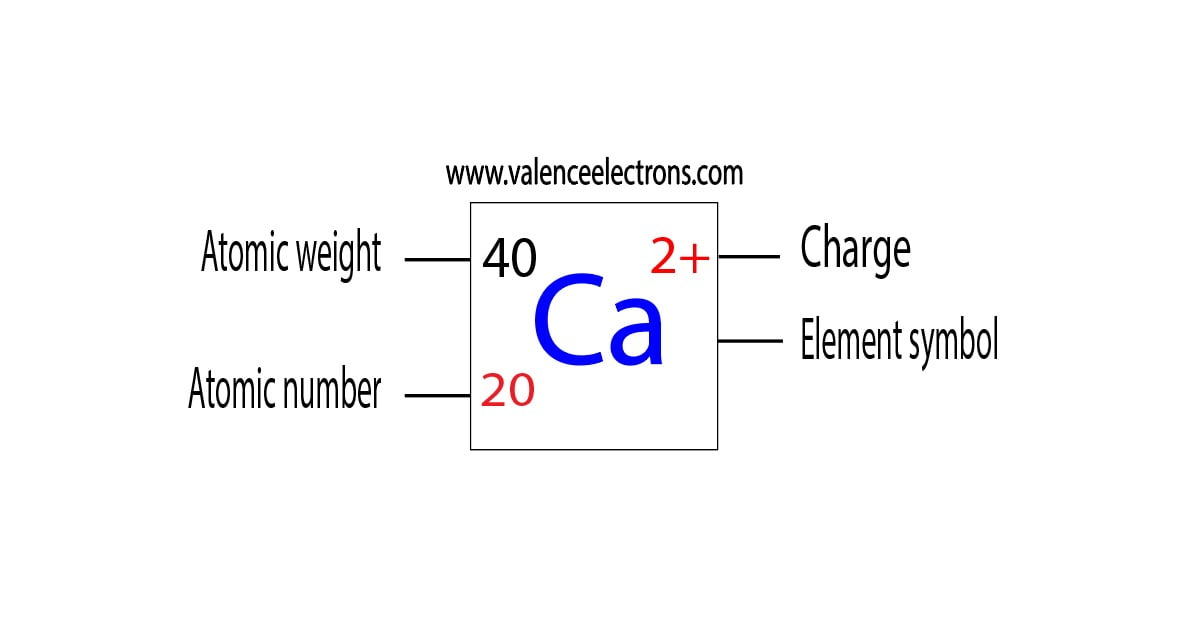
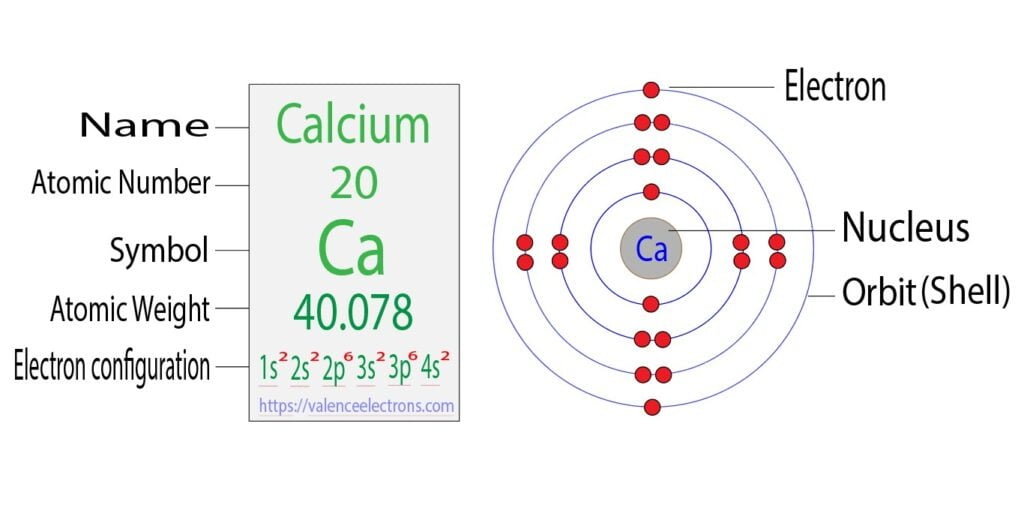
What Is The Electron Configuration Of The Calcium Ion - The calcium ion (ca 2+ ),. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). The electron configuration of a #ca^(2+)# ion. Simply use this information to obtain its electronic configuration. The electron configuration of the calcium ion (ca^2+) is: Let's determine the electron configuration of a calcium ion,. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. First, we need to know the electron configuration of a neutral. Calcium has an atomic number of 20. By removing the two outermost electrons from.
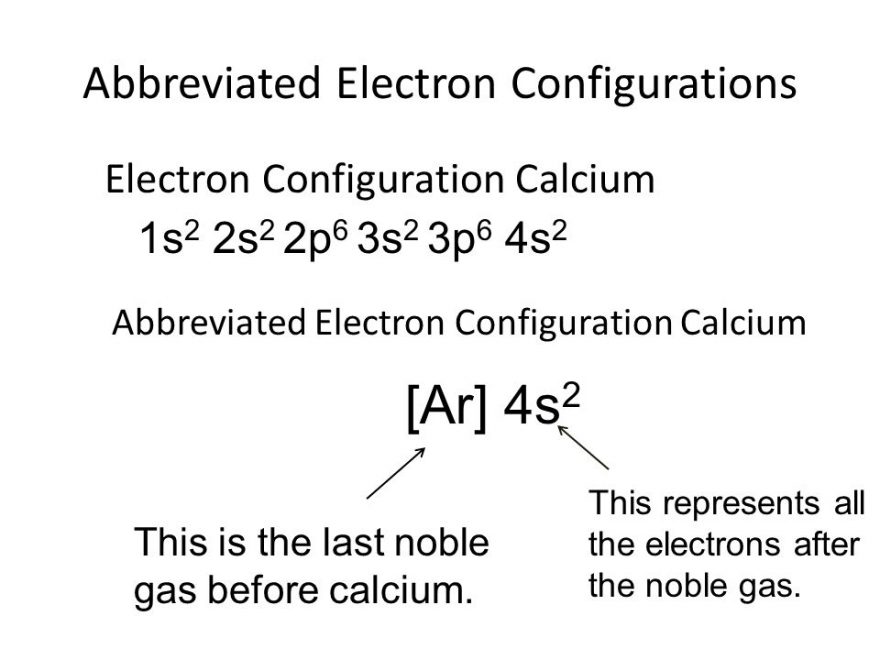
1s^2 2s^2 2p^6 3s^2 3p^6. By removing the two outermost electrons from. The electron configuration of the calcium ion (ca^2+) is: Simply use this information to obtain its electronic configuration. First, we need to know the electron configuration of a neutral. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. The calcium ion (ca 2+ ),. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The electron configuration of a #ca^(2+)# ion. Let's determine the electron configuration of a calcium ion,.
Let's determine the electron configuration of a calcium ion,. First, we need to know the electron configuration of a neutral. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Calcium has an atomic number of 20. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. The electron configuration of the calcium ion (ca^2+) is: 1s^2 2s^2 2p^6 3s^2 3p^6. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The electron configuration of a #ca^(2+)# ion. By removing the two outermost electrons from.
Electron Configuration For Calcium
Calcium has an atomic number of 20. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). The calcium ion (ca 2+ ),. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. Let's determine the electron configuration of a.
Electron Configuration For Calcium
The calcium ion (ca 2+ ),. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 1s^2 2s^2 2p^6 3s^2 3p^6. The electron configuration of the calcium ion (ca^2+) is:
Calcium Electron Configuration (Ca) with Orbital Diagram
Calcium has an atomic number of 20. Let's determine the electron configuration of a calcium ion,. 1s^2 2s^2 2p^6 3s^2 3p^6. The electron configuration of a #ca^(2+)# ion. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons).
Electron Configuration for Calcium (Ca, Ca2+ ion)
The electron configuration of a #ca^(2+)# ion. First, we need to know the electron configuration of a neutral. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium has an atomic number of 20. A calcium #2+# ion has lost its two valence electrons, and now.
Electron arrangements
For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The electron configuration of the calcium ion (ca^2+) is: First, we need to know the electron configuration of a neutral. Simply use this information to obtain its electronic configuration. 1s^2 2s^2 2p^6 3s^2 3p^6.
Electron Configuration For Calcium
Let's determine the electron configuration of a calcium ion,. 1s^2 2s^2 2p^6 3s^2 3p^6. The electron configuration of a #ca^(2+)# ion. Simply use this information to obtain its electronic configuration. Calcium has an atomic number of 20.
Calcium electron configuration Stock Image C029/5027 Science
1s^2 2s^2 2p^6 3s^2 3p^6. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). The calcium ion (ca 2+ ),. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Let's.
Orbital Diagram For Calcium (Ca) Calcium Electron Configuration
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). The electron configuration of the calcium ion (ca^2+) is: The electron configuration of a #ca^(2+)# ion. The calcium ion (ca 2+ ),. By removing the two outermost electrons from.
How to Write the Electron Configuration for Calcium (Ca)
By removing the two outermost electrons from. Let's determine the electron configuration of a calcium ion,. Simply use this information to obtain its electronic configuration. Calcium has an atomic number of 20. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
How to Write the Electron Configuration for Calcium (Ca)
For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Simply use this information to obtain its electronic configuration. The electron configuration of the calcium ion (ca^2+) is: By removing the two outermost electrons from. Calcium has an atomic number of 20.
A Calcium #2+# Ion Has Lost Its Two Valence Electrons, And Now Has #18# Electrons.
The electron configuration of a #ca^(2+)# ion. Let's determine the electron configuration of a calcium ion,. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. By removing the two outermost electrons from.
1S^2 2S^2 2P^6 3S^2 3P^6.
The calcium ion (ca 2+ ),. Simply use this information to obtain its electronic configuration. The electron configuration of the calcium ion (ca^2+) is: First, we need to know the electron configuration of a neutral.
Calcium Has An Atomic Number Of 20.
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons).