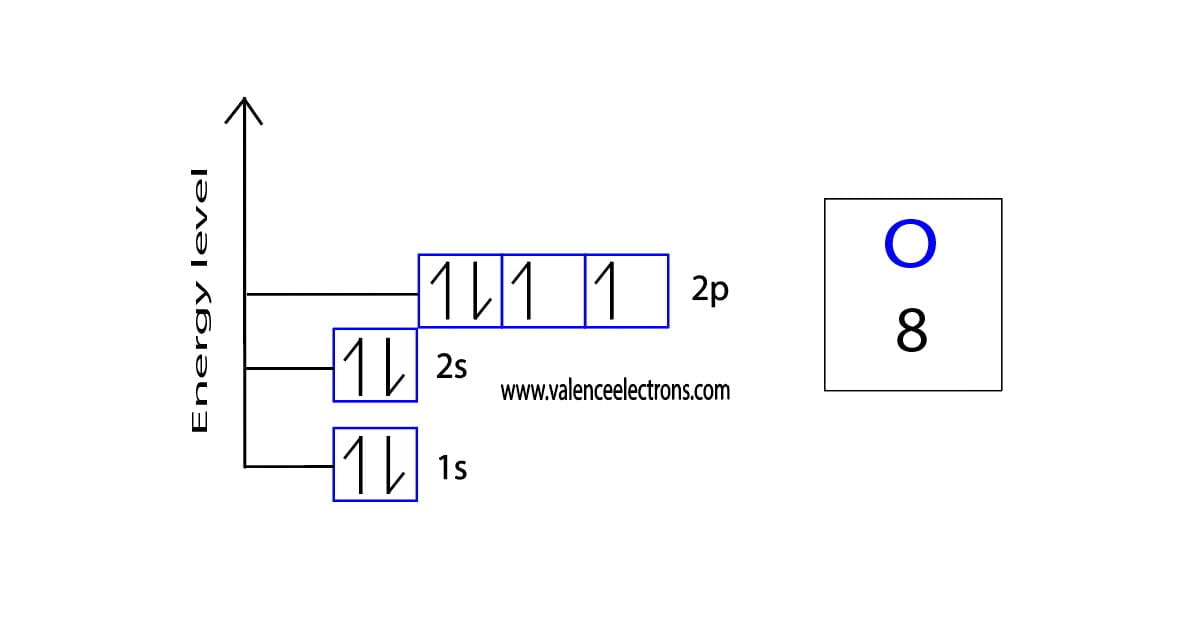
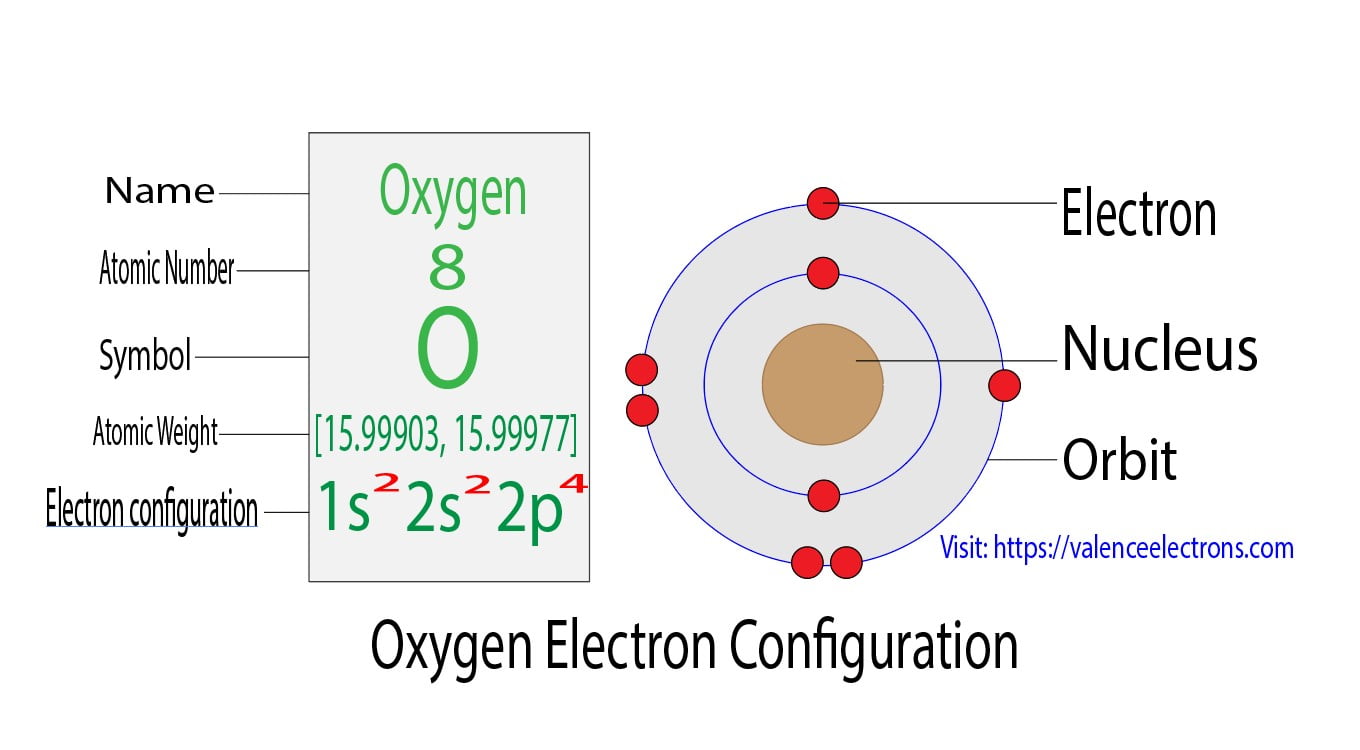
What Is The Electronic Configuration Of Oxygen - K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. The shorthand electron configuration (or noble gas. Atomic number of oxygen is 8. Electron configuration chart of all elements is mentioned in the table below. Oxygen atom contains eight electrons. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. Its electron arrangement can be written as k l 2 6 The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an.
Oxygen atom contains eight electrons. The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. Its electron arrangement can be written as k l 2 6 The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Atomic number of oxygen is 8.
Its electron arrangement can be written as k l 2 6 K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. Atomic number of oxygen is 8. Oxygen atom contains eight electrons. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas.
Electron configuration of oxygen ion Lousiana
Electron configuration chart of all elements is mentioned in the table below. K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. Oxygen atom contains eight electrons. Its electron arrangement can be written as k l 2 6 The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not.
Electronic configuration of the oxygen atom Download Scientific Diagram
Oxygen atom contains eight electrons. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Atomic number of oxygen is 8. Electron configuration chart of all elements is mentioned in the table below. Its electron arrangement can be written as k l 2 6
Oxygen Electron Configuration (O) with Orbital Diagram
Atomic number of oxygen is 8. Its electron arrangement can be written as k l 2 6 The shorthand electron configuration (or noble gas. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Electron configuration chart of all elements is mentioned in the table below.
Oxygen(O) electron configuration and orbital diagram (2022)
Electron configuration chart of all elements is mentioned in the table below. Oxygen atom contains eight electrons. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. The shorthand electron configuration (or noble.
Electron Configuration For Oxygen
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Atomic number of oxygen is 8. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. Oxygen atom contains eight electrons. Its electron arrangement can be.
Oxygen Electron Configuration (O) with Orbital Diagram
K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. Electron configuration chart of all elements is mentioned in the table below. Atomic number of oxygen is 8. Oxygen atom contains eight electrons. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2.
How to Write the Electron Configuration for Oxygen (O)
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Atomic number of oxygen is 8. Oxygen atom contains eight electrons. The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. K shell can accommodate 2.
Electron Configuration for Oxygen (O, O2 ion)
Oxygen atom contains eight electrons. K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. Its electron arrangement can be written as k l 2 6 Atomic number of oxygen is 8. Electron configuration chart of all elements is mentioned in the table below.
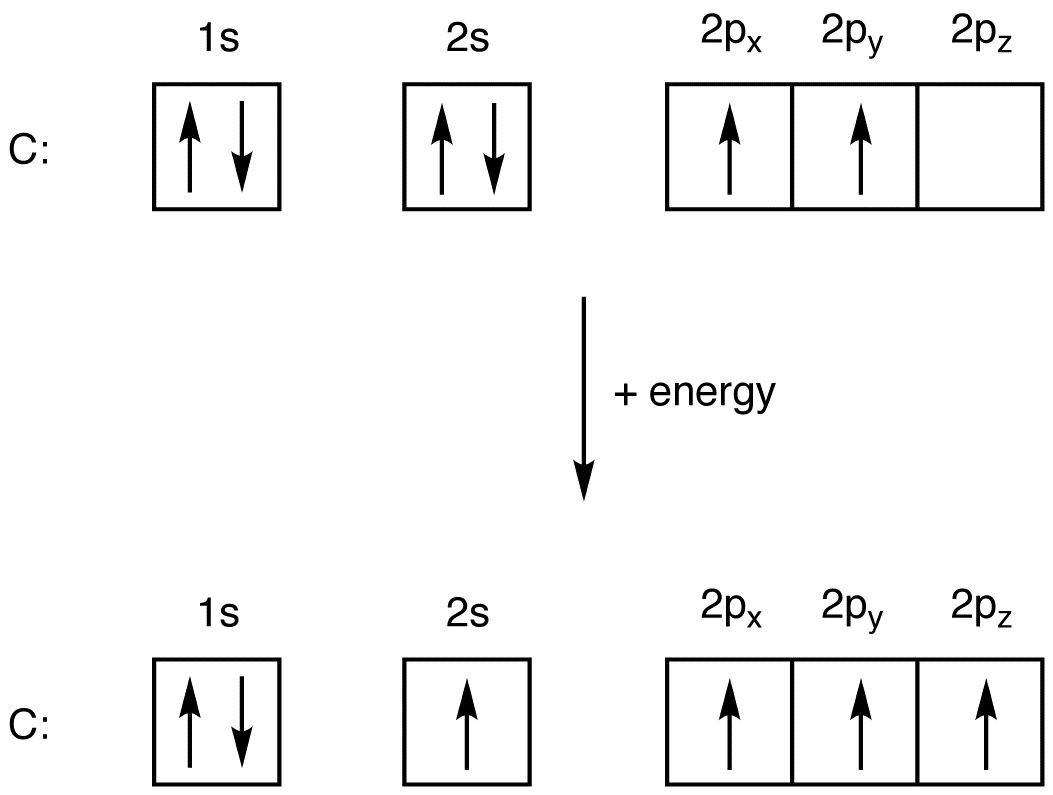
(iii) Oxygen molecule, O2 The electronic configuration of oxygen atom O=..
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Oxygen atom contains eight electrons. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. Atomic number of oxygen is 8.
Electron configuration of oxygen ion Lousiana
Electron configuration chart of all elements is mentioned in the table below. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons. The electronic configuration of oxygen is written as 1s 2 2s.
The Shorthand Electron Configuration (Or Noble Gas.
Electron configuration chart of all elements is mentioned in the table below. Atomic number of oxygen is 8. Its electron arrangement can be written as k l 2 6 K shell can accommodate 2 electrons and l shell can accommodate the remaining 6 electrons.
The Configuration Notation Provides An Easy Way For Scientists To Write And Communicate How Electrons Are Arranged Around The Nucleus Of An.
The electronic configuration of oxygen is written as 1s 2 2s 2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s 2 2s 2. Oxygen atom contains eight electrons.