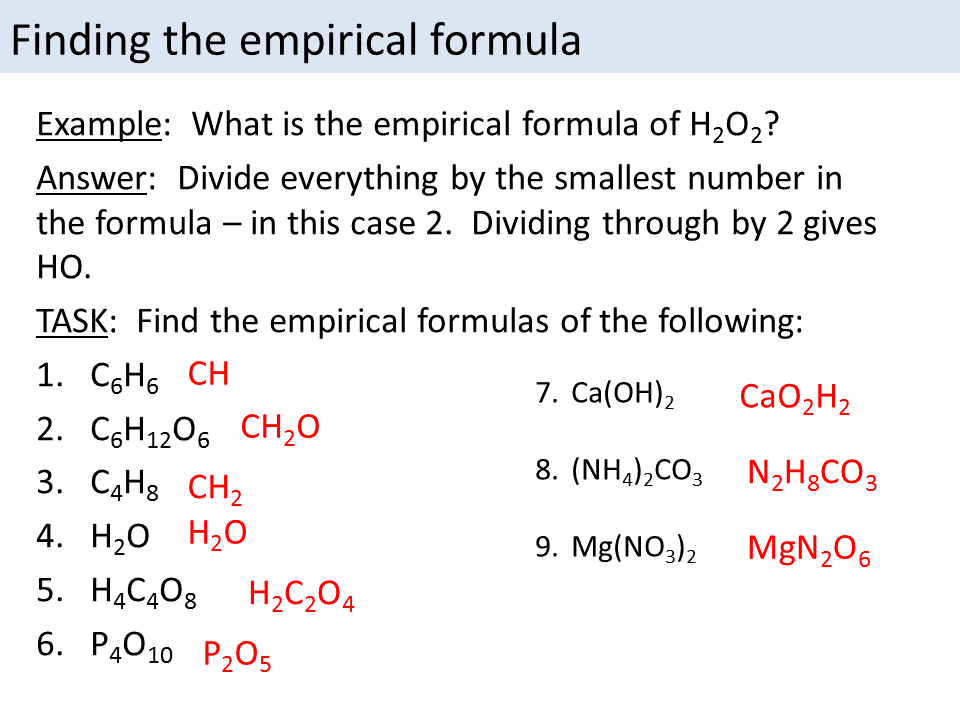
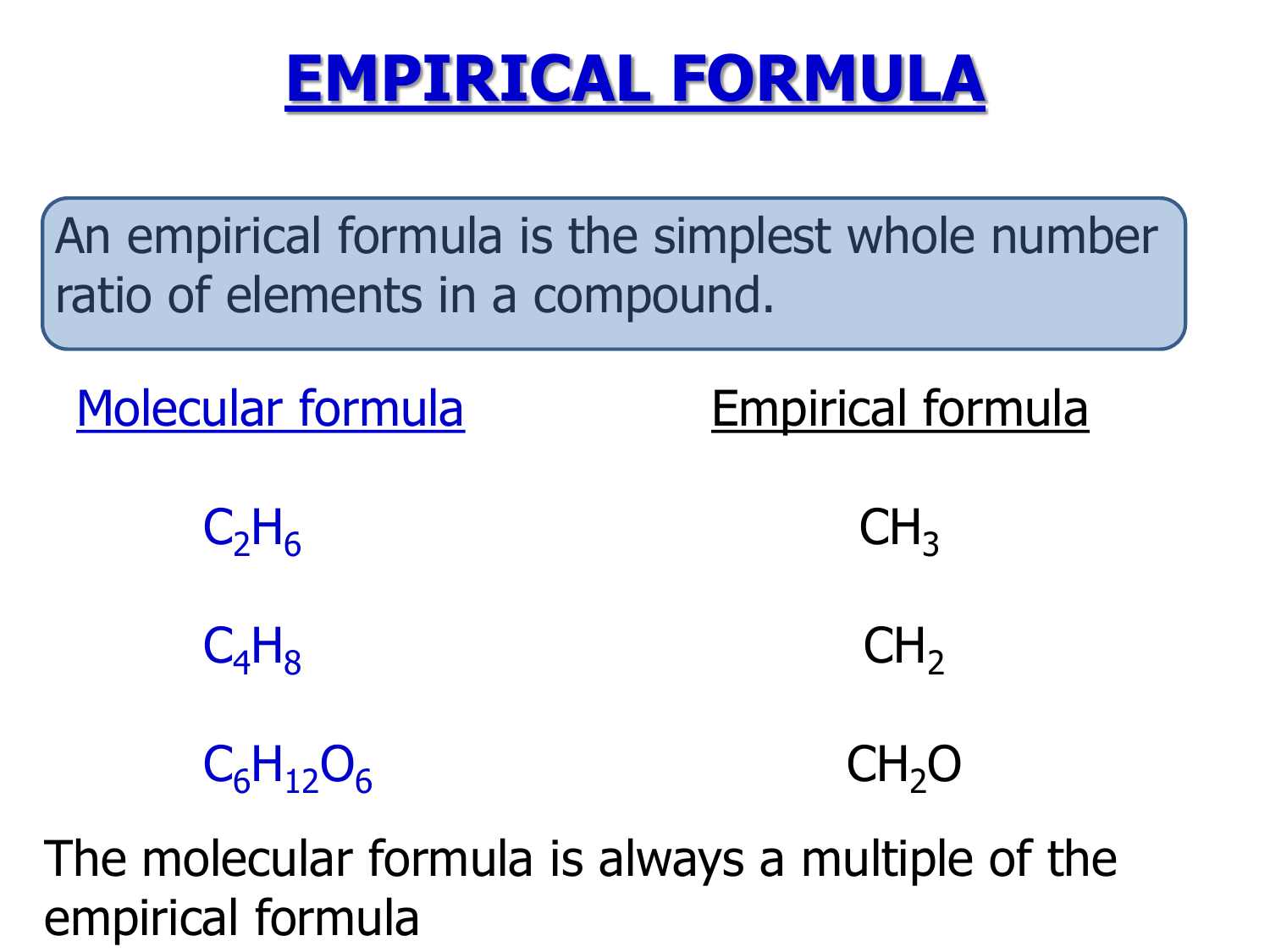
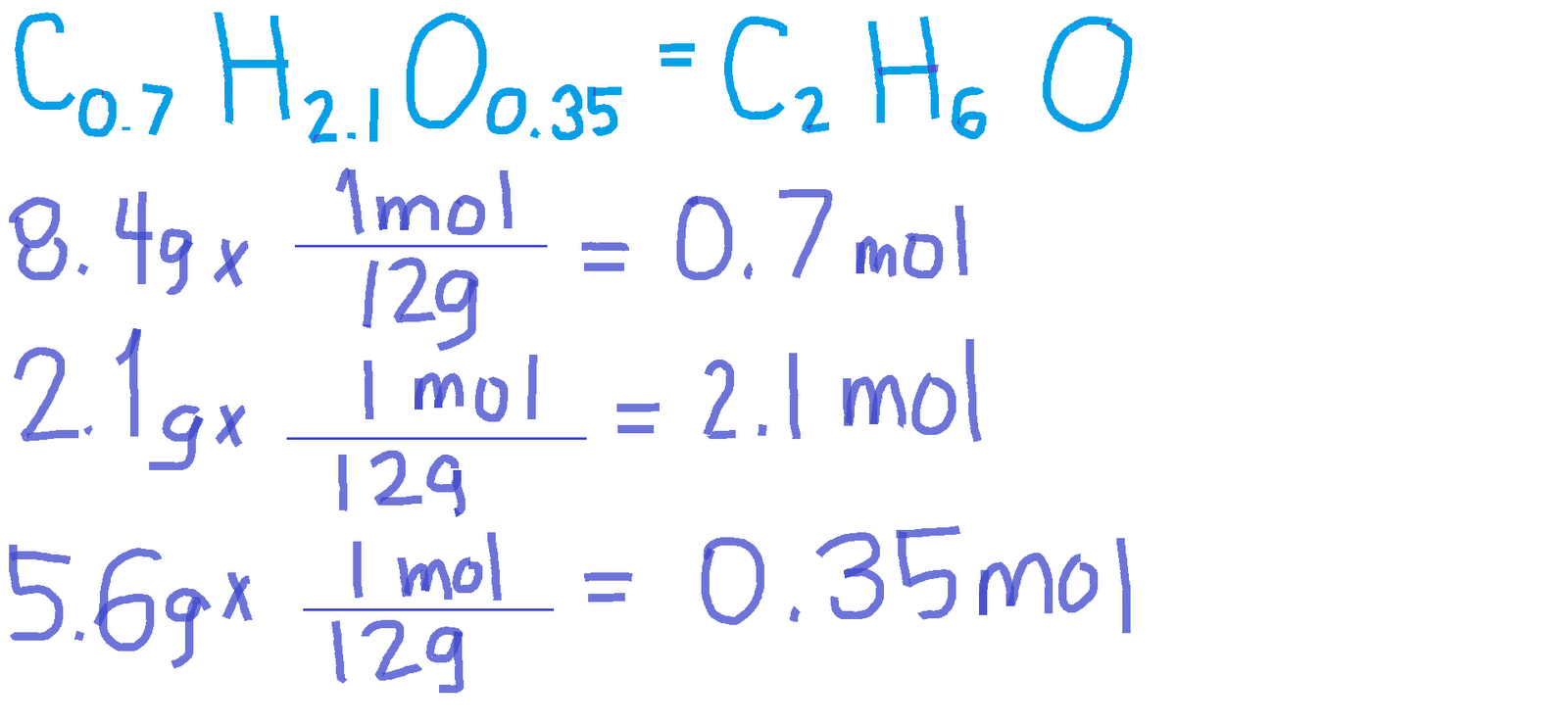What Is The Empirical Formula For C6H6 - Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen. 0.2 g atom of silicon combine with 21.3 g of chlorine. Find the empirical formula of the compound formed. The empirical formula is the simplest whole number ratio defining constituent atoms in a. Explain the term empirical formula. A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen. The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. Benzene molecular formula ≡ c6h 6. For (a) c6h6, the simplified form is ch,. The empirical formula represents the simplest whole number ratio of atoms of all the elements present in a compound.
The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. Explain the term empirical formula. A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen. The empirical formula is the simplest whole number ratio defining constituent atoms in a. Benzene molecular formula ≡ c6h 6. Its molecular formula is c6h6. The empirical formula expresses the fundamental ratio of elements in a compound. Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen. Empirical formula is different from the molecular formula. For (a) c6h6, the simplified form is ch,.
Its molecular formula is c6h6. Find the empirical formula of the compound formed. The empirical formula is the simplest whole number ratio defining constituent atoms in a. Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen. For (a) c6h6, the simplified form is ch,. 0.2 g atom of silicon combine with 21.3 g of chlorine. The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. The empirical formula expresses the fundamental ratio of elements in a compound. Explain the term empirical formula. Benzene molecular formula ≡ c6h 6.
Empirical Formula Table
The empirical formula represents the simplest whole number ratio of atoms of all the elements present in a compound. The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. Find the empirical formula of the compound formed. Its molecular formula is c6h6. The empirical formula is the simplest whole.
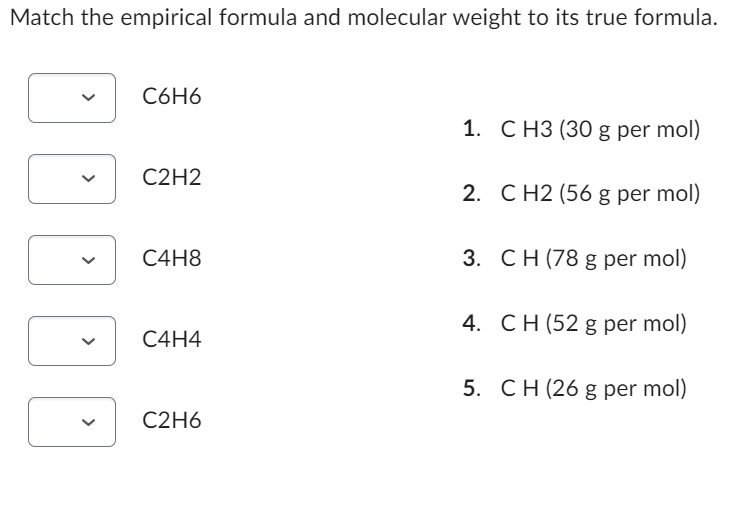
Solved Match the empirical formula and molecular weight to
The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. Benzene molecular formula ≡ c6h 6. Empirical formula is different from the molecular formula. Explain the term empirical formula. The empirical formula represents the simplest whole number ratio of atoms of all the elements present in a compound.
CHEMISTRY 11 EMPIRICAL&MOLECULAR FORMULAS
Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen. Explain the term empirical formula. The empirical formula represents the simplest whole number ratio of atoms of all the elements present in a compound. The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. A compound contains 87.5% by mass of.
Find Empirical Formula From Equation Tessshebaylo
Empirical formula is different from the molecular formula. Benzene molecular formula ≡ c6h 6. Its molecular formula is c6h6. For (a) c6h6, the simplified form is ch,. Explain the term empirical formula.
SOLVED The Mass of Hydrogen Atoms Exercise 1.4 Empirical and
The empirical formula expresses the fundamental ratio of elements in a compound. 0.2 g atom of silicon combine with 21.3 g of chlorine. Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen. A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen. Benzene molecular formula ≡ c6h 6.
empirical formula
For (a) c6h6, the simplified form is ch,. 0.2 g atom of silicon combine with 21.3 g of chlorine. The empirical formula represents the simplest whole number ratio of atoms of all the elements present in a compound. Find the empirical formula of the compound formed. The empirical formula is the simplest whole number ratio defining constituent atoms in a.
Write the empirical formula of each of the following substances. Part A
Explain the term empirical formula. The empirical formula is the simplest whole number ratio defining constituent atoms in a. Empirical formula is different from the molecular formula. The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. Find the empirical formula of the compound formed.
SOLVED 1. How do you distinguish between molecular formula, empirical
0.2 g atom of silicon combine with 21.3 g of chlorine. For (a) c6h6, the simplified form is ch,. Explain the term empirical formula. The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen.
Ch.10 Empirical and Molecular Formula Worksheet
The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. The empirical formula expresses the fundamental ratio of elements in a compound. Empirical formula is different from the molecular formula. Benzene molecular formula ≡ c6h 6. Its molecular formula is c6h6.
Empirical and Molecular Formula Practice by Teach Simple
Its molecular formula is c6h6. Empirical formula is different from the molecular formula. 0.2 g atom of silicon combine with 21.3 g of chlorine. Find the empirical formula of the compound formed. Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen.
Empirical Formula Is Different From The Molecular Formula.
The empirical formula for benzene (c6h6) is ch, representing a 1:1 ratio of carbon to hydrogen atoms in its simplest form. The empirical formula expresses the fundamental ratio of elements in a compound. The empirical formula represents the simplest whole number ratio of atoms of all the elements present in a compound. A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen.
Explain The Term Empirical Formula.
Its molecular formula is c6h6. The empirical formula is the simplest whole number ratio defining constituent atoms in a. Find the empirical formula of the compound formed. Benzene molecular formula ≡ c6h 6.
0.2 G Atom Of Silicon Combine With 21.3 G Of Chlorine.
For (a) c6h6, the simplified form is ch,. Octaethylporphyrin is comprised of carbon, hydrogen, and nitrogen.