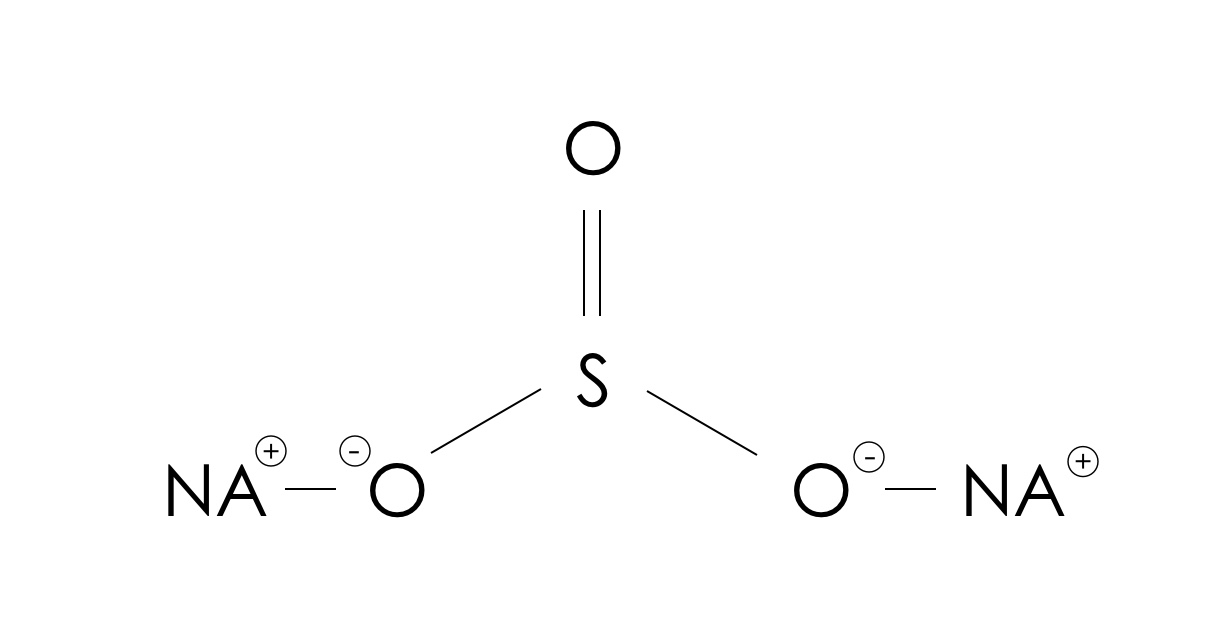
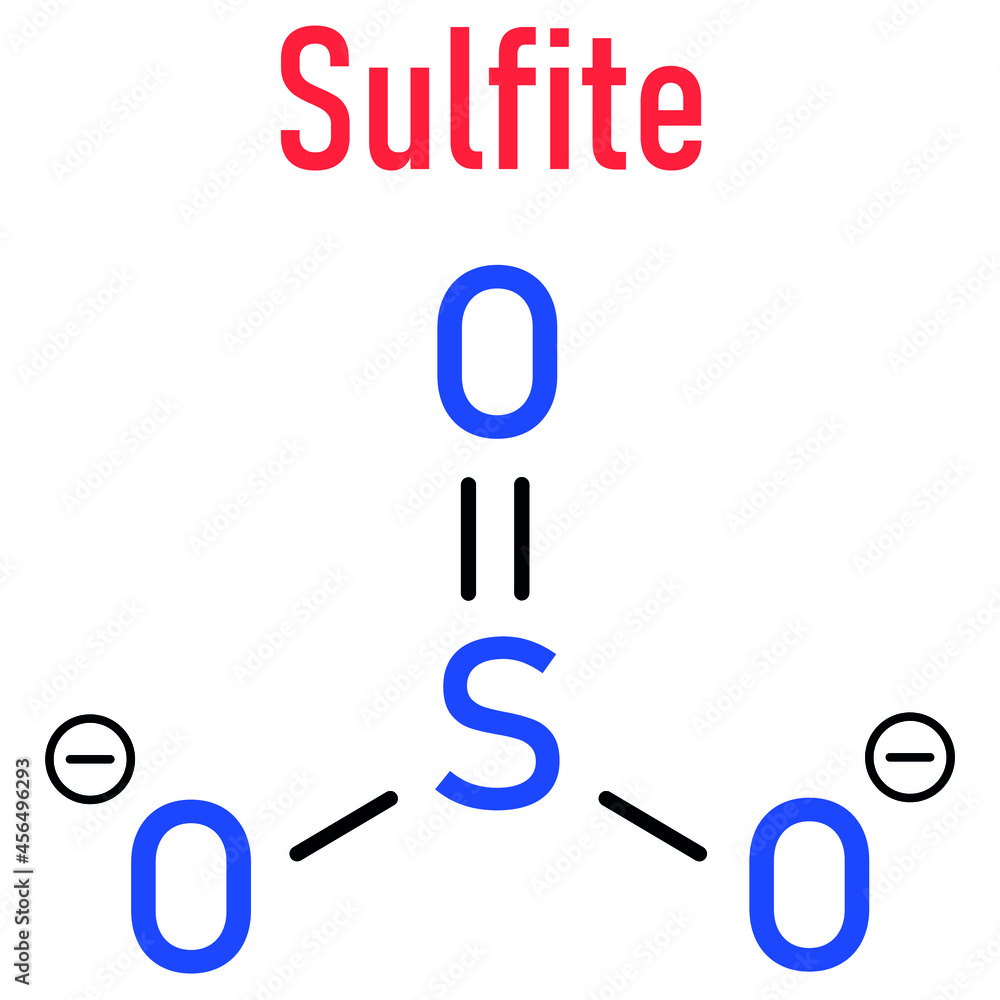
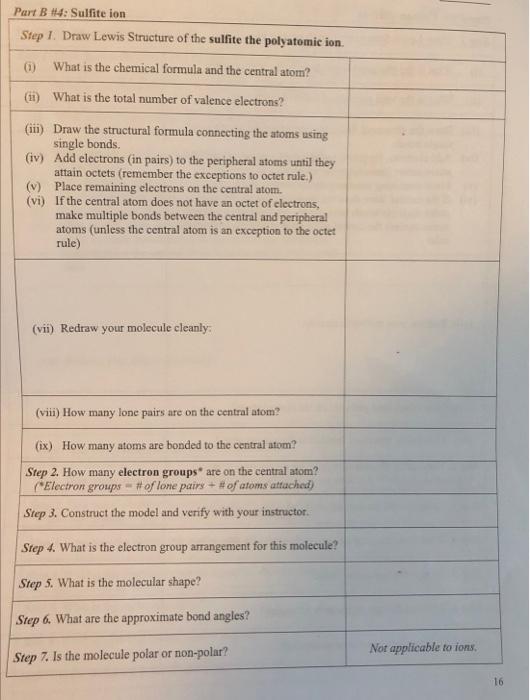
What Is The Formula For The Sulfite Ion - The correct formula for the sulfite ion is so₃²⁻. Answer choice (c) is correct. It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The ion consists of a sulfur atom surrounded by three oxygen atoms, with an. The chemical formula for the sulfite ion is so3−². The formula is so 3 2 −. Identify the components of the sulfite ion. The sulfite ion is a polyatomic anion with one sulfur.
The chemical formula for the sulfite ion is so3−². Identify the components of the sulfite ion. Answer choice (c) is correct. The ion consists of a sulfur atom surrounded by three oxygen atoms, with an. The correct formula for the sulfite ion is so₃²⁻. The sulfite ion is a polyatomic anion with one sulfur. It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The formula is so 3 2 −.
The formula is so 3 2 −. Answer choice (c) is correct. The chemical formula for the sulfite ion is so3−². It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The sulfite ion is a polyatomic anion with one sulfur. The correct formula for the sulfite ion is so₃²⁻. Identify the components of the sulfite ion. The ion consists of a sulfur atom surrounded by three oxygen atoms, with an.
Sulfite Molecule, Structural Chemical Formula, Ballandstick Model
The formula is so 3 2 −. Identify the components of the sulfite ion. The correct formula for the sulfite ion is so₃²⁻. Answer choice (c) is correct. The sulfite ion is a polyatomic anion with one sulfur.
Sulfite Ion Stock Illustrations 23 Sulfite Ion Stock Illustrations
It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The correct formula for the sulfite ion is so₃²⁻. The sulfite ion is a polyatomic anion with one sulfur. Answer choice (c) is correct. Identify the components of the sulfite ion.
(Get Answer) 20) What is the chemical formula for the sulfite ion? A
Identify the components of the sulfite ion. The sulfite ion is a polyatomic anion with one sulfur. The chemical formula for the sulfite ion is so3−². The correct formula for the sulfite ion is so₃²⁻. The formula is so 3 2 −.
SODIUM SULFITE PHOTO GRADE Esseco USA
The chemical formula for the sulfite ion is so3−². The sulfite ion is a polyatomic anion with one sulfur. The correct formula for the sulfite ion is so₃²⁻. Answer choice (c) is correct. The formula is so 3 2 −.
Sulfite anion, chemical structure. Sulfite salts are common food
It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The formula is so 3 2 −. The sulfite ion is a polyatomic anion with one sulfur. Answer choice (c) is correct. The correct formula for the sulfite ion is so₃²⁻.
Molecular Formula Chemical Structure Sulfite Stock Vector (Royalty Free
Identify the components of the sulfite ion. It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The formula is so 3 2 −. The ion consists of a sulfur atom surrounded by three oxygen atoms, with an. The sulfite ion is a polyatomic anion with one sulfur.
Sulfite Molecule, Structural Chemical Formula, Ballandstick Model
Identify the components of the sulfite ion. The chemical formula for the sulfite ion is so3−². Answer choice (c) is correct. The formula is so 3 2 −. The ion consists of a sulfur atom surrounded by three oxygen atoms, with an.
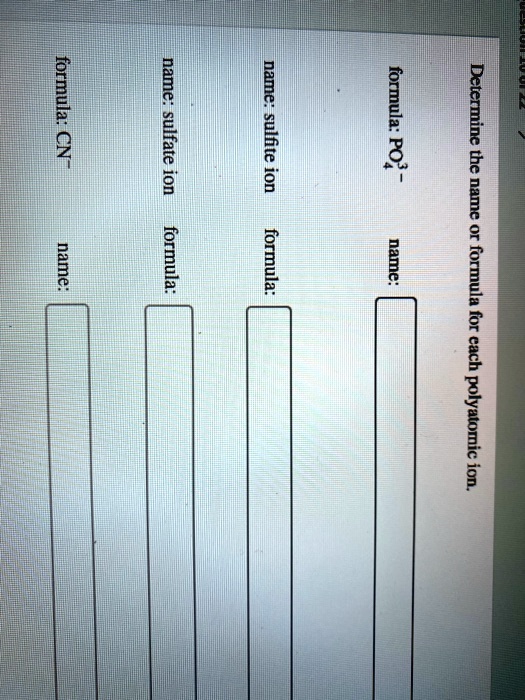
SOLVED formula CN name sulfate ion name sulfite ion name formula
The sulfite ion is a polyatomic anion with one sulfur. The correct formula for the sulfite ion is so₃²⁻. The formula is so 3 2 −. It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. Answer choice (c) is correct.
Sulfite Molecular Structure, 3d Model Molecule, Sulfite Ion, Structural
The formula is so 3 2 −. Identify the components of the sulfite ion. Answer choice (c) is correct. The sulfite ion is a polyatomic anion with one sulfur. The correct formula for the sulfite ion is so₃²⁻.
The Sulfite Ion Is A Polyatomic Anion With One Sulfur.
It consists of one sulfur atom bonded to four oxygen atoms in a tetrahedral arrangement. The formula is so 3 2 −. Identify the components of the sulfite ion. Answer choice (c) is correct.
The Chemical Formula For The Sulfite Ion Is So3−².
The ion consists of a sulfur atom surrounded by three oxygen atoms, with an. The correct formula for the sulfite ion is so₃²⁻.