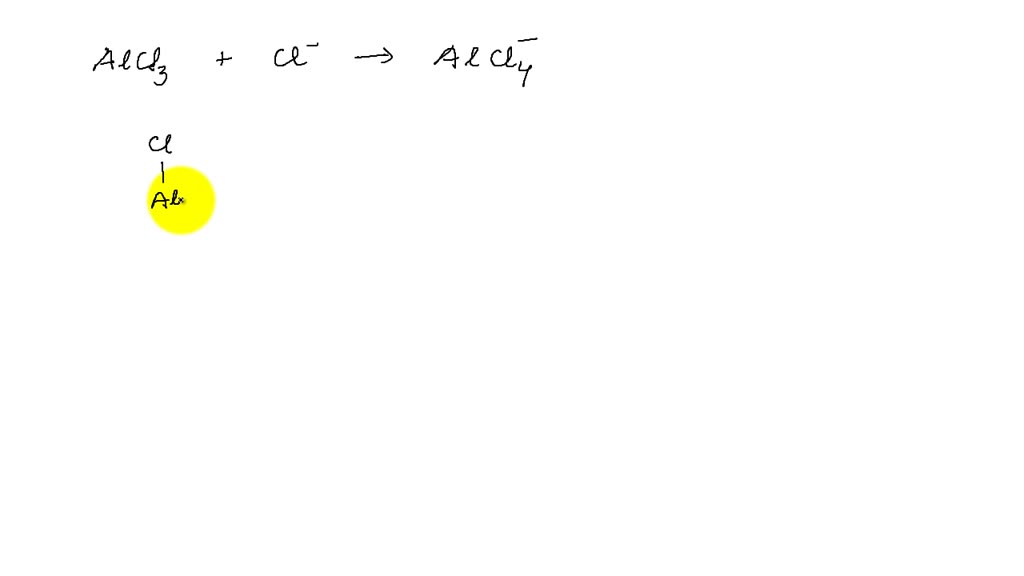What Is The Hybridization Of The Central Atom In Alcl3 - The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Name a molecule having a banana bond. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. Draw the structure of the following. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom.
Name a molecule having a banana bond. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. Draw the structure of the following. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom.
Draw the structure of the following. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. Name a molecule having a banana bond. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms.
Answered What is the hybridization of the… bartleby
The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of.
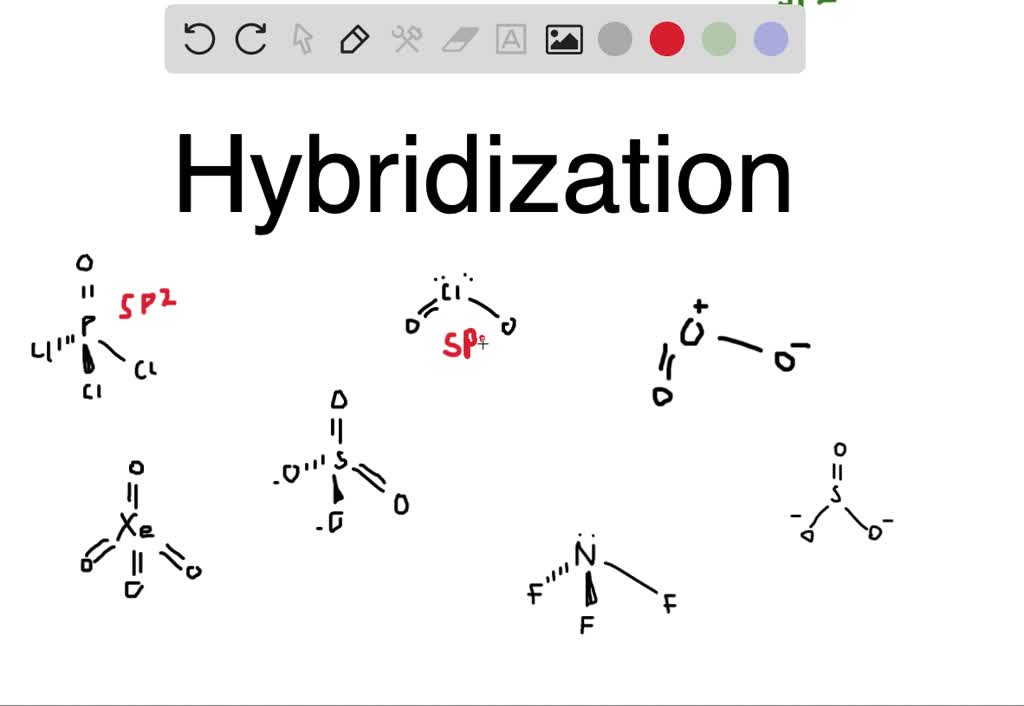
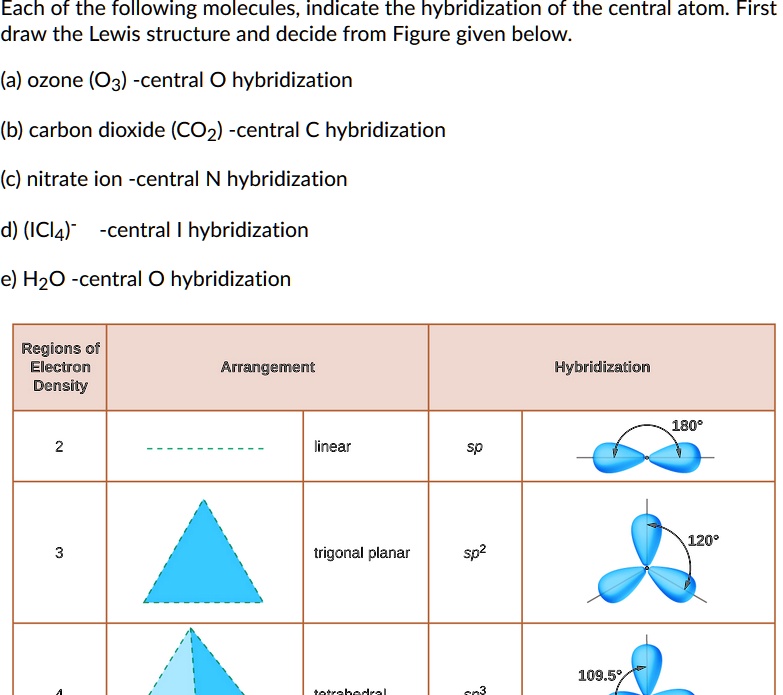
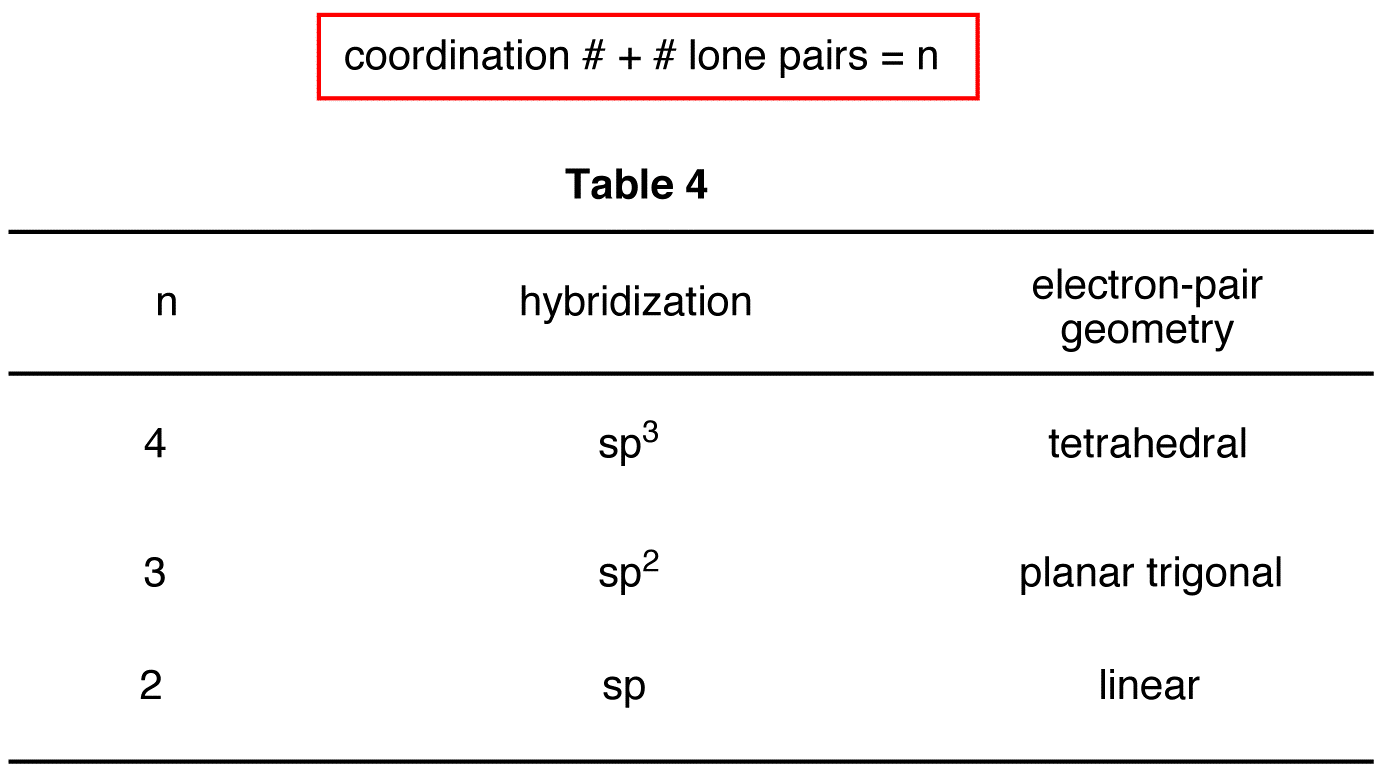
Each of the following molecules indicates the hybridization of the
Draw the structure of the following. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. Name a molecule having a banana bond. The al2cl6 dimer has a central al atom.
Hybridization Chart
Draw the structure of the following. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and.
Hybridization Chart
Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Name a molecule having a banana bond. Draw the structure of the following. The al2cl6 dimer has a central al atom.
SOLVEDDescribe the change in hybridization (if any) of the Al atom in
The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. Draw the structure of the following. Acl 3 comprises a single, central aluminum atom that forms.
SOLVED For the molecule AlCl3, identify the hybridization of aluminum
Name a molecule having a banana bond. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. The central atom in alcl3, aluminum, exhibits sp².
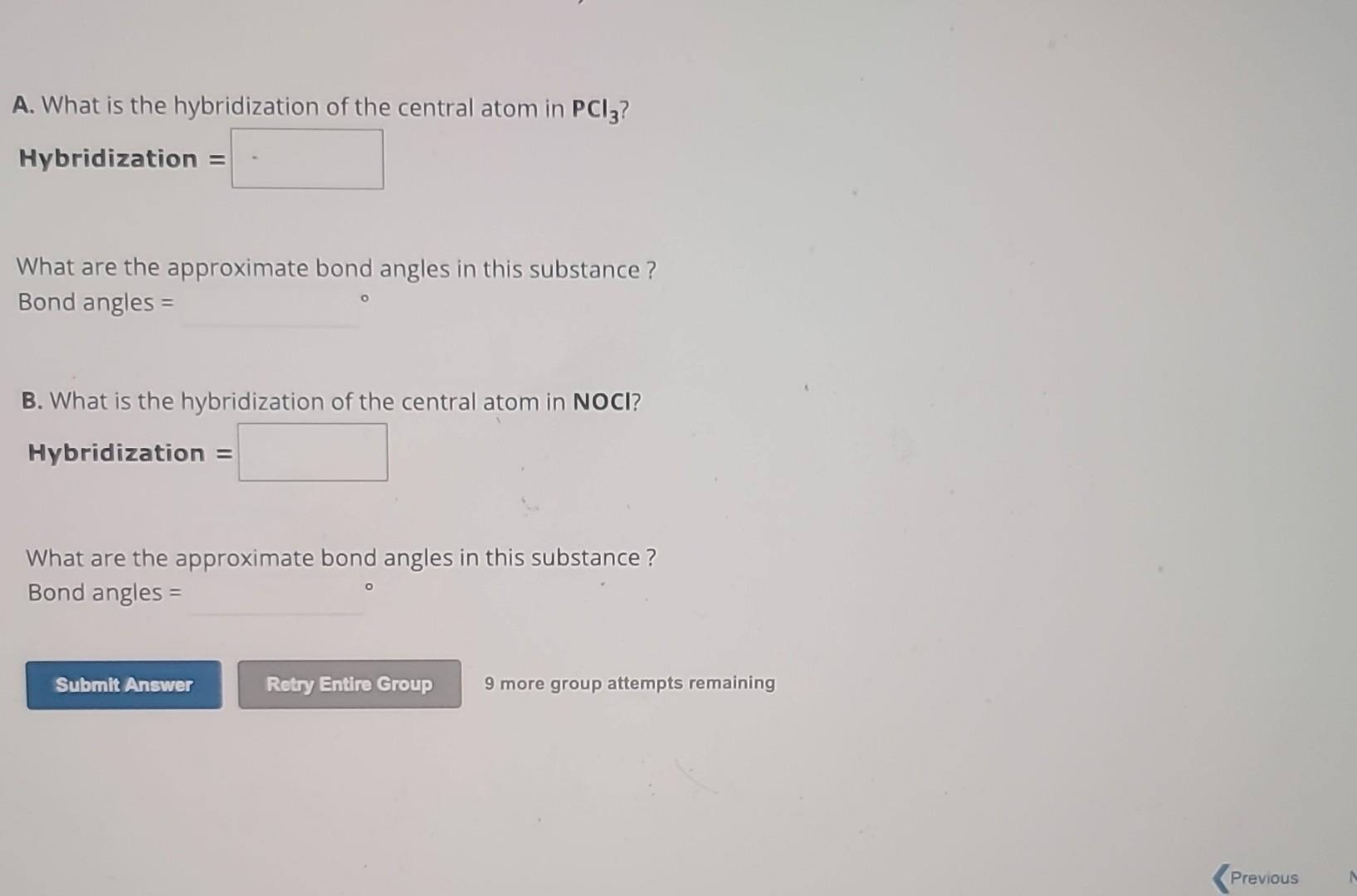
Solved A. What is the hybridization of the central atom in
Name a molecule having a banana bond. Draw the structure of the following. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. The central.
SOLVEDIn which of the following charye, the hybridization state of
The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. Draw the structure of the following. The central atom in alcl3, aluminum, exhibits sp² hybridization,.
SOLVED Homework Chapter 9 Bonding Orbitals 1. Give the expected
Name a molecule having a banana bond. Acl 3 comprises a single, central aluminum atom that forms covalent bonds with a set of three surrounding chlorine atoms. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Draw the structure of the following. The al2cl6 dimer has a central al atom.
SOLVED Give the expected hybridization of the central atom for the
Name a molecule having a banana bond. The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Acl 3 comprises a single, central aluminum atom that.
Acl 3 Comprises A Single, Central Aluminum Atom That Forms Covalent Bonds With A Set Of Three Surrounding Chlorine Atoms.
The al2cl6 dimer has a central al atom bonded to two terminal cl atoms and two bridging cl atoms shared with the other al atom. The central atom in alcl3, aluminum, exhibits sp² hybridization, due to it being surrounded by three regions of electron. Name a molecule having a banana bond. Draw the structure of the following.

2]++4.+Tetrahedral.+sp3.+[Cd(NH3)4]2+.jpg)