What Is The Hybridization Of The Central Atom In Bef2 - Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of. In bef2, the central atom beryllium has sp hybridisation. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. Since be has two bonds and no lone pairs, its hybridization is. Beryllium has two valence electrons which pair up to form two. The central atom in bef2 is be. What is the hybridization of bf2? The sp2 hybrid of the unpaired electron on the b has 44% 2s character and 56% 2p character. The hybridization of the central beryllium atom in bef2 is sp.
The sp2 hybrid of the unpaired electron on the b has 44% 2s character and 56% 2p character. In bef2, the central atom beryllium has sp hybridisation. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. The hybridization of the central beryllium atom in bef2 is sp. Since be has two bonds and no lone pairs, its hybridization is. The central atom in bef2 is be. What is the hybridization of bf2? Beryllium has two valence electrons which pair up to form two. Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of.
Since be has two bonds and no lone pairs, its hybridization is. Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of. The hybridization of the central beryllium atom in bef2 is sp. In bef2, the central atom beryllium has sp hybridisation. What is the hybridization of bf2? To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. The central atom in bef2 is be. The sp2 hybrid of the unpaired electron on the b has 44% 2s character and 56% 2p character. Beryllium has two valence electrons which pair up to form two.
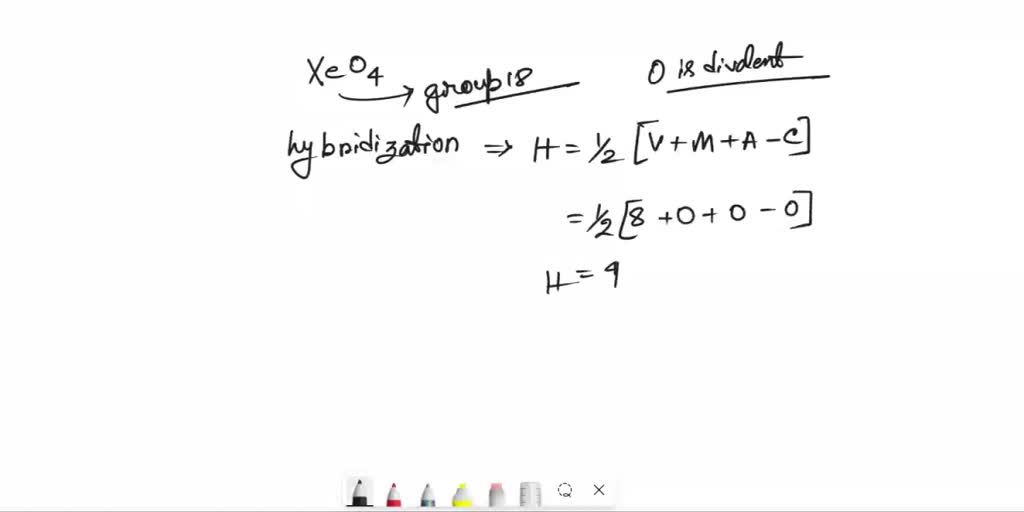
SOLVED What is the hybridization of the central atom in XeO4? What are
The central atom in bef2 is be. Beryllium has two valence electrons which pair up to form two. The hybridization of the central beryllium atom in bef2 is sp. Since be has two bonds and no lone pairs, its hybridization is. What is the hybridization of bf2?
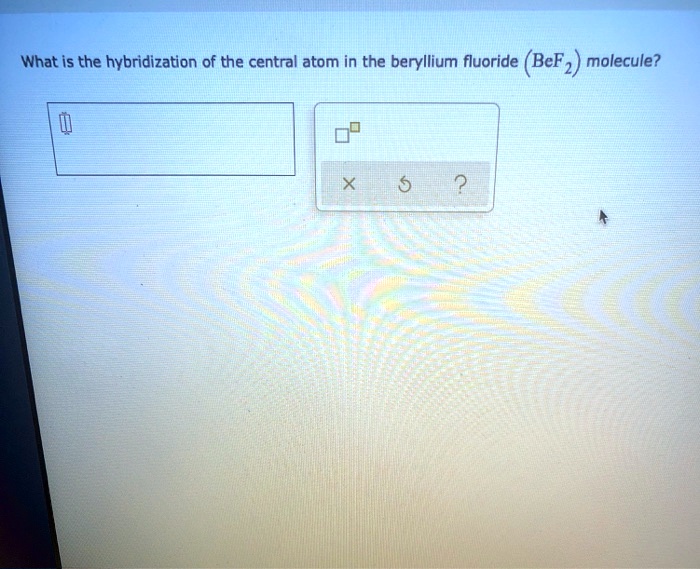
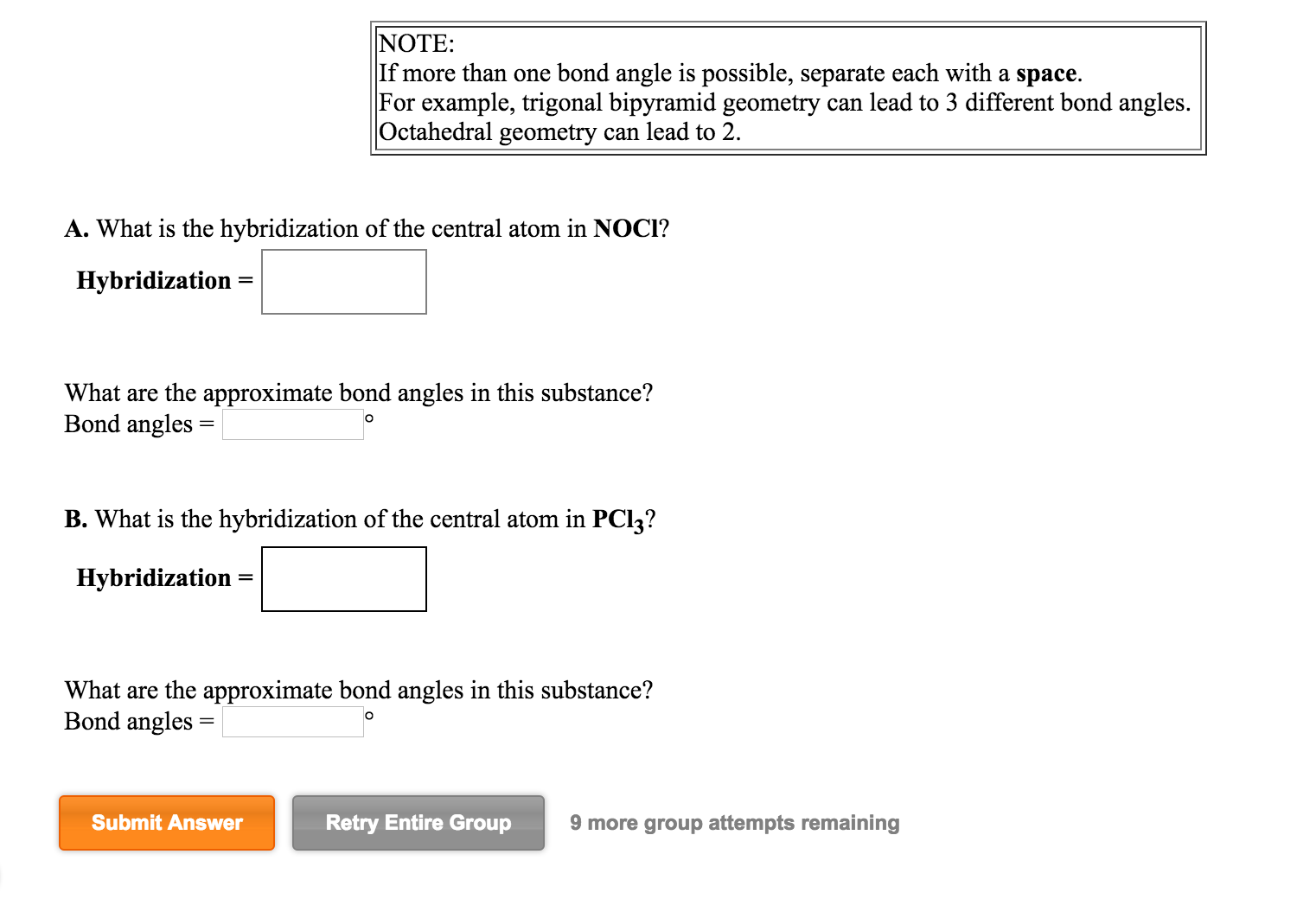
Solved A. What is the hybridization of the central atom in
Since be has two bonds and no lone pairs, its hybridization is. The sp2 hybrid of the unpaired electron on the b has 44% 2s character and 56% 2p character. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. The hybridization of the central beryllium atom.
Solved A. What is the hybridization of the central atom in
Since be has two bonds and no lone pairs, its hybridization is. The hybridization of the central beryllium atom in bef2 is sp. What is the hybridization of bf2? Beryllium has two valence electrons which pair up to form two. In bef2, the central atom beryllium has sp hybridisation.
SOLVED What is the hybridization of the central atom in the beryllium
To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. In bef2, the central atom beryllium has sp hybridisation. Since be has two bonds and no lone pairs, its hybridization is. Beryllium has two valence electrons which pair up to form two. The central atom in bef2.
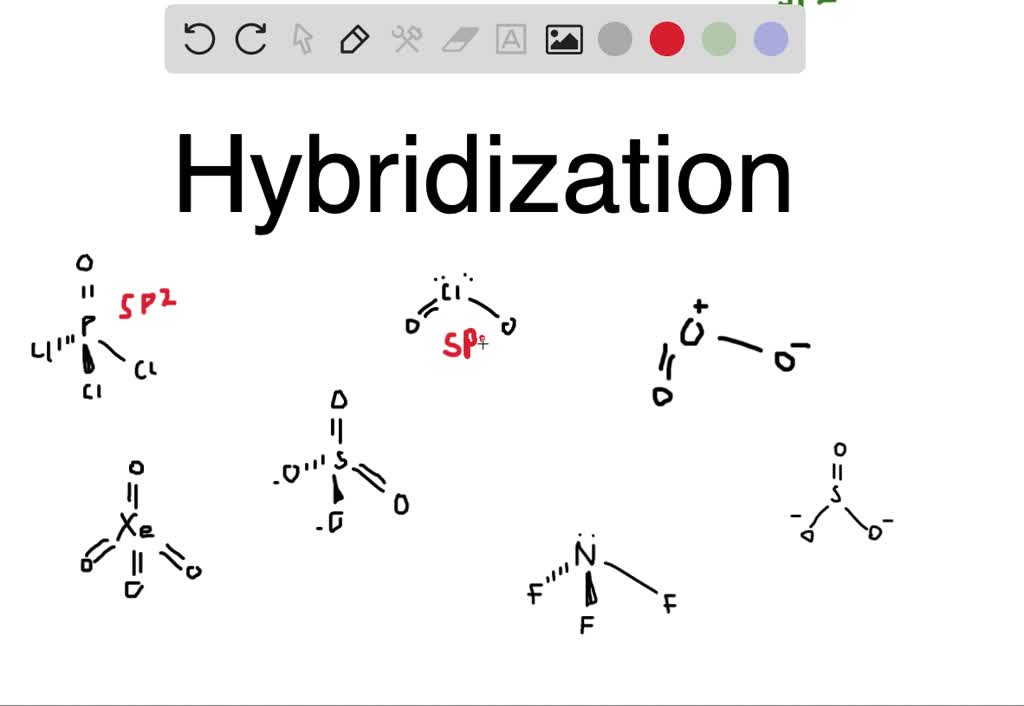
SOLVED Give the expected hybridization of the central atom for the
Since be has two bonds and no lone pairs, its hybridization is. The hybridization of the central beryllium atom in bef2 is sp. The sp2 hybrid of the unpaired electron on the b has 44% 2s character and 56% 2p character. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the.
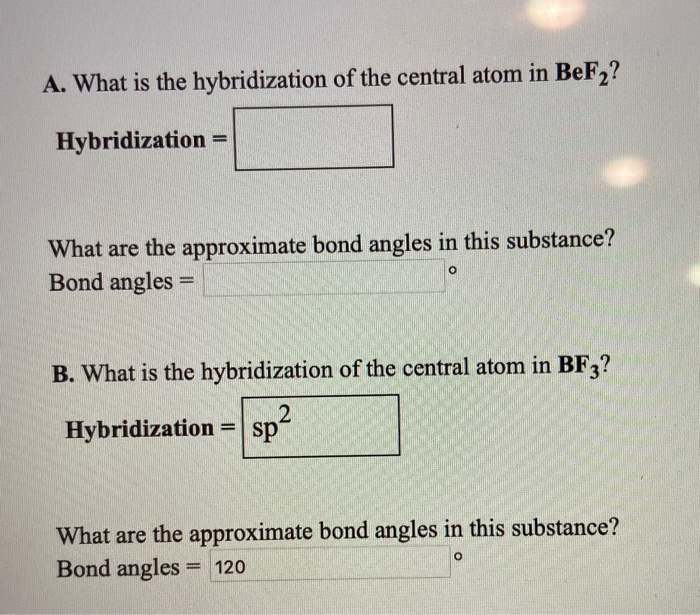
Solved A. What is the hybridization of the central atom in
What is the hybridization of bf2? Beryllium has two valence electrons which pair up to form two. Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of. The hybridization of the central beryllium atom in bef2 is sp. The sp2 hybrid of the unpaired electron on the b.
Answered A. What is the hybridization of the… bartleby
To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of. The sp2 hybrid of the unpaired electron on the b has 44% 2s character and.
Solved A. What is the hybridization of the central atom in
Beryllium has two valence electrons which pair up to form two. Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of. The central atom in bef2 is be. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding.
SOLVED Lewis Structure and hybridization of central atom BeF2 Sketch
What is the hybridization of bf2? The central atom in bef2 is be. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. The hybridization of the central beryllium atom in bef2 is sp. Since be has two bonds and no lone pairs, its hybridization is.
Solved What is the hybridization of the central atom in
To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. The hybridization of the central beryllium atom in bef2 is sp. Since be has two bonds and no lone pairs, its hybridization is. What is the hybridization of bf2? The sp2 hybrid of the unpaired electron on.
What Is The Hybridization Of Bf2?
The hybridization of the central beryllium atom in bef2 is sp. Beryllium has two valence electrons which pair up to form two. Hybridisation :hybridisation, is a phenomenon of intermixing of atomic orbitals of almost equal energy which are present in the outer shells of. In bef2, the central atom beryllium has sp hybridisation.
The Central Atom In Bef2 Is Be.
Since be has two bonds and no lone pairs, its hybridization is. To find the hybridization of the central atom in beryllium fluoride (bef₂), we need to look at how the bonding and structure work. The sp2 hybrid of the unpaired electron on the b has 44% 2s character and 56% 2p character.